Hepatocyte Injury and Hepatic Stem Cell Niche in the Progression of Non-Alcoholic Steatohepatitis
- PMID: 32131439
- PMCID: PMC7140508
- DOI: 10.3390/cells9030590
Hepatocyte Injury and Hepatic Stem Cell Niche in the Progression of Non-Alcoholic Steatohepatitis
Abstract
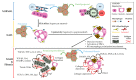
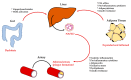
Non-alcoholic fatty liver disease (NAFLD) is a chronic liver disease characterized by lipid accumulation in hepatocytes in the absence of excessive alcohol consumption. The global prevalence of NAFLD is constantly increasing. NAFLD is a disease spectrum comprising distinct stages with different prognoses. Non-alcoholic steatohepatitis (NASH) is a progressive condition, characterized by liver inflammation and hepatocyte ballooning, with or without fibrosis. The natural history of NAFLD is negatively influenced by NASH onset and by the progression towards advanced fibrosis. Pathogenetic mechanisms and cellular interactions leading to NASH and fibrosis involve hepatocytes, liver macrophages, myofibroblast cell subpopulations, and the resident progenitor cell niche. These cells are implied in the regenerative trajectories following liver injury, and impairment or perturbation of these mechanisms could lead to NASH and fibrosis. Recent evidence underlines the contribution of extra-hepatic organs/tissues (e.g., gut, adipose tissue) in influencing NASH development by interacting with hepatic cells through various molecular pathways. The present review aims to summarize the role of hepatic parenchymal and non-parenchymal cells, their mutual influence, and the possible interactions with extra-hepatic tissues and organs in the pathogenesis of NAFLD.
Keywords: adipose tissue; atherosclerosis; disease; ductular reaction; fibrosis; lipotoxicity; liver; macrophage; progenitor cell; regeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Single-Cell Data Analysis Reveals Critical Hepatic Cells Subpopulations in the Progression of Non-alcoholic Fatty Liver Disease to Non-Alcoholic Steatohepatitis.Comb Chem High Throughput Screen. 2025;28(7):1251-1263. doi: 10.2174/0113862073303213240523095742. Comb Chem High Throughput Screen. 2025. PMID: 38803181
-
CD44 is a key player in non-alcoholic steatohepatitis.J Hepatol. 2017 Aug;67(2):328-338. doi: 10.1016/j.jhep.2017.03.003. Epub 2017 Mar 16. J Hepatol. 2017. PMID: 28323124
-
The transcription factor c-Jun/AP-1 promotes liver fibrosis during non-alcoholic steatohepatitis by regulating Osteopontin expression.Cell Death Differ. 2019 Sep;26(9):1688-1699. doi: 10.1038/s41418-018-0239-8. Epub 2019 Feb 18. Cell Death Differ. 2019. PMID: 30778201 Free PMC article.
-
Metabolic drivers of non-alcoholic fatty liver disease.Mol Metab. 2021 Aug;50:101143. doi: 10.1016/j.molmet.2020.101143. Epub 2020 Dec 17. Mol Metab. 2021. PMID: 33346069 Free PMC article. Review.
-
Chronic Inflammation in Non-Alcoholic Steatohepatitis: Molecular Mechanisms and Therapeutic Strategies.Front Endocrinol (Lausanne). 2020 Dec 14;11:597648. doi: 10.3389/fendo.2020.597648. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33384662 Free PMC article. Review.
Cited by
-
Polyphenol-Rich Extracts of Xylopia and Aframomum Species Show Metabolic Benefits by Lowering Hepatic Lipid Accumulation in Diet-Induced Obese Mice.ACS Omega. 2022 Mar 30;7(14):11914-11928. doi: 10.1021/acsomega.2c00050. eCollection 2022 Apr 12. ACS Omega. 2022. PMID: 35449947 Free PMC article.
-
Cell transplantation-based regenerative medicine in liver diseases.Stem Cell Reports. 2023 Aug 8;18(8):1555-1572. doi: 10.1016/j.stemcr.2023.06.005. Stem Cell Reports. 2023. PMID: 37557073 Free PMC article. Review.
-
Non-alcoholic fatty liver disease and gut microbial dysbiosis- underlying mechanisms and gut microbiota mediated treatment strategies.Rev Endocr Metab Disord. 2023 Dec;24(6):1189-1204. doi: 10.1007/s11154-023-09843-z. Epub 2023 Oct 16. Rev Endocr Metab Disord. 2023. PMID: 37840104 Review.
-
Label-free metabolic imaging of non-alcoholic-fatty-liver-disease (NAFLD) liver by volumetric dynamic optical coherence tomography.Biomed Opt Express. 2022 Jun 30;13(7):4071-4086. doi: 10.1364/BOE.461433. eCollection 2022 Jul 1. Biomed Opt Express. 2022. PMID: 35991915 Free PMC article.
-
Decoding the hepatic fibrosis-hepatocellular carcinoma axis: from mechanisms to therapeutic opportunities.Hepatol Int. 2025 Aug;19(4):732-759. doi: 10.1007/s12072-025-10838-y. Epub 2025 Jul 1. Hepatol Int. 2025. PMID: 40588713 Review.
References
-
- European Association for the Study of the Liver. European Association for the Study of Diabetes. European Association for the Study of Obesity EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. - DOI - PubMed
-
- Estes C., Anstee Q.M., Arias-Loste M.T., Bantel H., Bellentani S., Caballeria J., Colombo M., Craxi A., Crespo J., Day C.P., et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 2018;69:896–904. doi: 10.1016/j.jhep.2018.05.036. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

