Beneficial and Detrimental Effects of Regulatory T Cells in Neurotropic Virus Infections
- PMID: 32131483
- PMCID: PMC7084400
- DOI: 10.3390/ijms21051705
Beneficial and Detrimental Effects of Regulatory T Cells in Neurotropic Virus Infections
Abstract
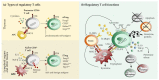
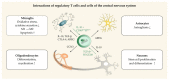
Neurotropic viruses infect the central nervous system (CNS) and cause acute or chronic neurologic disabilities. Regulatory T cells (Treg) play a critical role for immune homeostasis, but may inhibit pathogen-specific immunity in infectious disorders. The present review summarizes the current knowledge about Treg in human CNS infections and their animal models. Besides dampening pathogen-induced immunopathology, Treg have the ability to facilitate protective responses by supporting effector T cell trafficking to the infection site and the development of resident memory T cells. Moreover, Treg can reduce virus replication by inducing apoptosis of infected macrophages and attenuate neurotoxic astrogliosis and pro-inflammatory microglial responses. By contrast, detrimental effects of Treg are caused by suppression of antiviral immunity, allowing for virus persistence and latency. Opposing disease outcomes following Treg manipulation in different models might be attributed to differences in technique and timing of intervention, infection route, genetic background, and the host's age. In addition, mouse models of virus-induced demyelination revealed that Treg are able to reduce autoimmunity and immune-mediated CNS damage in a disease phase-dependent manner. Understanding the unique properties of Treg and their complex interplay with effector cells represents a prerequisite for the development of new therapeutic approaches in neurotropic virus infections.
Keywords: Foxp3; animal models; central nervous system; demyelination; neuroinflammation; neurotropic viruses; regulatory T cells.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Foxp3(+) regulatory T cells, immune stimulation and host defence against infection.Immunology. 2012 May;136(1):1-10. doi: 10.1111/j.1365-2567.2011.03551.x. Immunology. 2012. PMID: 22211994 Free PMC article. Review.
-
Dynamic changes of Foxp3(+) regulatory T cells in spleen and brain of canine distemper virus-infected dogs.Vet Immunol Immunopathol. 2013 Dec 15;156(3-4):215-22. doi: 10.1016/j.vetimm.2013.10.006. Epub 2013 Oct 16. Vet Immunol Immunopathol. 2013. PMID: 24210687
-
One, No One, and One Hundred Thousand: T Regulatory Cells' Multiple Identities in Neuroimmunity.Front Immunol. 2019 Dec 20;10:2947. doi: 10.3389/fimmu.2019.02947. eCollection 2019. Front Immunol. 2019. PMID: 31956323 Free PMC article. Review.
-
[The role of regulatory cells CD4+CD25+ in the development of chronic infective diseases].Vestn Ross Akad Med Nauk. 2006;(9-10):24-9. Vestn Ross Akad Med Nauk. 2006. PMID: 17111920 Review. Russian.
-
The role of regulatory T cells in nervous system pathologies.J Neurosci Res. 2018 Jun;96(6):951-968. doi: 10.1002/jnr.24073. Epub 2017 May 10. J Neurosci Res. 2018. PMID: 28488363 Review.
Cited by
-
Balancing functions of regulatory T cells in mosquito-borne viral infections.Emerg Microbes Infect. 2024 Dec;13(1):2304061. doi: 10.1080/22221751.2024.2304061. Epub 2024 Jan 25. Emerg Microbes Infect. 2024. PMID: 38192073 Free PMC article. Review.
-
The Role of Herpes Simplex Virus Type 1 Infection in Demyelination of the Central Nervous System.Int J Mol Sci. 2020 Jul 16;21(14):5026. doi: 10.3390/ijms21145026. Int J Mol Sci. 2020. PMID: 32708697 Free PMC article. Review.
-
Correlates of disease severity in bluetongue as a model of acute arbovirus infection.PLoS Pathog. 2024 Aug 16;20(8):e1012466. doi: 10.1371/journal.ppat.1012466. eCollection 2024 Aug. PLoS Pathog. 2024. PMID: 39150989 Free PMC article.
-
Balanced T and B cell responses are required for immune protection against Powassan virus in virus-like particle vaccination.Cell Rep. 2022 Feb 15;38(7):110388. doi: 10.1016/j.celrep.2022.110388. Cell Rep. 2022. PMID: 35172138 Free PMC article.
-
Usutu virus-induced meningoencephalitis in immunocompetent mice is characterized by the recruitment of mononuclear cells and a proinflammatory T helper 1 response.J Virol. 2025 Mar 18;99(3):e0172424. doi: 10.1128/jvi.01724-24. Epub 2025 Feb 5. J Virol. 2025. PMID: 39907280 Free PMC article.
References
-
- Broer S., Hage E., Kaufer C., Gerhauser I., Anjum M., Li L., Baumgärtner W., Schulz T.F., Loscher W. Viral mouse models of multiple sclerosis and epilepsy: Marked differences in neuropathogenesis following infection with two naturally occurring variants of Theiler’s virus BeAn strain. Neurobiol. disease. 2017;99:121–132. doi: 10.1016/j.nbd.2016.12.020. - DOI - PubMed
-
- Ludlow M., Kortekaas J., Herden C., Hoffmann B., Tappe D., Trebst C., Griffin D.E., Brindle H.E., Solomon T., Brown A.S., et al. Neurotropic virus infections as the cause of immediate and delayed neuropathology. Acta. Neuropathol. 2016;131:159–184. doi: 10.1007/s00401-015-1511-3. - DOI - PMC - PubMed
-
- Sun X., Hua S., Chen H.R., Ouyang Z., Einkauf K., Tse S., Ard K., Ciaranello A., Yawetz S., Sax P., et al. Transcriptional Changes during Naturally Acquired Zika Virus Infection Render Dendritic Cells Highly Conducive to Viral Replication. Cell Rep. 2017;21:3471–3482. doi: 10.1016/j.celrep.2017.11.087. - DOI - PMC - PubMed
-
- Martines R.B., Bhatnagar J., de Oliveira Ramos A.M., Davi H.P., Iglezias S.D., Kanamura C.T., Keating M.K., Hale G., Silva-Flannery L., Muehlenbachs A., et al. Pathology of congenital Zika syndrome in Brazil: A case series. Lancet. 2016;388:898–904. doi: 10.1016/S0140-6736(16)30883-2. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

