Fourier-Domain OCT Imaging of the Ocular Surface and Tear Film Dynamics: A Review of the State of the Art and an Integrative Model of the Tear Behavior During the Inter-Blink Period and Visual Fixation
- PMID: 32131486
- PMCID: PMC7141198
- DOI: 10.3390/jcm9030668
Fourier-Domain OCT Imaging of the Ocular Surface and Tear Film Dynamics: A Review of the State of the Art and an Integrative Model of the Tear Behavior During the Inter-Blink Period and Visual Fixation
Abstract
In the last few decades, the ocular surface and the tear film have been noninvasively investigated in vivo, in a three-dimensional, high resolution, and real-time mode, by optical coherence tomography (OCT). Recently, OCT technology has made great strides in improving the acquisition speed and image resolution, thus increasing its impact in daily clinical practice and in the research setting. All these results have been achieved because of a transition from traditional time-domain (TD) to Fourier-domain (FD) technology. FD-OCT devices include a spectrometer in the receiver that analyzes the spectrum of reflected light on the retina or ocular surface and transforms it into information about the depth of the structures according to the Fourier principle. In this review, we summarize and provide the state-of-the-art in FD-OCT imaging of the ocular surface system, addressing specific aspects such as tear film dynamics and epithelial changes under physiologic and pathologic conditions. A theory on the dynamic nature of the tear film has been developed to explain the variations within the individual compartments. Moreover, an integrative model of tear film behavior during the inter-blink period and visual fixation is proposed.
Keywords: ocular surface; optical coherence tomography; tear film; tear film dynamics; visual fixation.
Conflict of interest statement
Pietro Emanuele Napoli, Matteo Nioi, Lorenzo Mangoni, Pietro Gentile, Mirco Braghiroli, Ernesto d’Aloja, Maurizio Fossarello– none to declare. No conflicting relationship exists for any author.
Figures


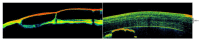
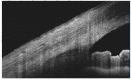

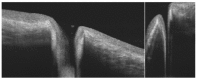
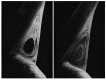

Similar articles
-
The diagnostic significance of Fourier-domain optical coherence tomography in Sjögren syndrome, aqueous tear deficiency and lipid tear deficiency patients.Acta Ophthalmol. 2012 Aug;90(5):e359-66. doi: 10.1111/j.1755-3768.2012.02413.x. Epub 2012 May 8. Acta Ophthalmol. 2012. PMID: 22568661
-
Spectral-domain optical coherence tomography study on dynamic changes of human tears after instillation of artificial tears.Invest Ophthalmol Vis Sci. 2014 Jul 1;55(7):4533-40. doi: 10.1167/iovs.14-14666. Invest Ophthalmol Vis Sci. 2014. PMID: 24985473
-
Measurement of a multi-layered tear film phantom using optical coherence tomography and statistical decision theory.Biomed Opt Express. 2014 Nov 24;5(12):4374-86. doi: 10.1364/BOE.5.004374. eCollection 2014 Dec 1. Biomed Opt Express. 2014. PMID: 25574445 Free PMC article.
-
Dynamics and function of the tear film in relation to the blink cycle.Prog Retin Eye Res. 2015 Mar;45:132-64. doi: 10.1016/j.preteyeres.2014.11.001. Epub 2014 Dec 3. Prog Retin Eye Res. 2015. PMID: 25479602 Free PMC article. Review.
-
Anterior segment optical coherence tomography.Prog Retin Eye Res. 2018 Sep;66:132-156. doi: 10.1016/j.preteyeres.2018.04.002. Epub 2018 Apr 7. Prog Retin Eye Res. 2018. PMID: 29635068 Review.
Cited by
-
Association Between Anterior Chamber Angle and Corneal Endothelial Cell Density in Chronic Angle Closure.Clin Ophthalmol. 2021 May 10;15:1957-1964. doi: 10.2147/OPTH.S309005. eCollection 2021. Clin Ophthalmol. 2021. PMID: 34007148 Free PMC article.
-
The Effect of Lockdown Due to the COVID-19 Pandemic on Digital Eye Strain Symptoms Among the General Population: A Cross-Sectional Survey.Front Public Health. 2022 Jun 22;10:895517. doi: 10.3389/fpubh.2022.895517. eCollection 2022. Front Public Health. 2022. PMID: 35812520 Free PMC article.
-
Understanding risks of refractive error among Chinese children amidst pandemic disruptions: results from a rapid survey.BMC Ophthalmol. 2021 Oct 19;21(1):370. doi: 10.1186/s12886-021-02133-9. BMC Ophthalmol. 2021. PMID: 34663261 Free PMC article.
-
Comparison of Different Types of Corneal Foreign Bodies Using Anterior Segment Optical Coherence Tomography: A Prospective Observational Study.J Ophthalmol. 2020 Aug 11;2020:9108317. doi: 10.1155/2020/9108317. eCollection 2020. J Ophthalmol. 2020. PMID: 32850143 Free PMC article.
-
Corneal Epithelial Changes in Diabetic Patients: A Review.Int J Mol Sci. 2024 Mar 19;25(6):3471. doi: 10.3390/ijms25063471. Int J Mol Sci. 2024. PMID: 38542443 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

