Halogen gas exposure: toxic effects on the parturient
- PMID: 32131668
- PMCID: PMC7547547
- DOI: 10.1080/15376516.2020.1736702
Halogen gas exposure: toxic effects on the parturient
Abstract
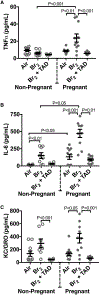
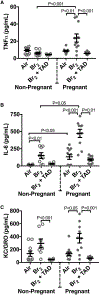
The elemental halogens include chlorine, bromine, and phosgene. Halogen gas can be directly weaponized and employed in warfare or terrorism. Industrial stockpiles or halogen transport can provide targets for terrorist attack as well as an origin for accidental release creating a risk for potential mass-casualty incidents. Pregnant and post-partum women represent a substantial and vulnerable subset of the population who may be at particular risk during an attack or accidental exposure. We review the effects of halogen exposure on the parturient with a focus on bromine toxicity. Bromine is the most extensively studied agent in the context of pregnancy and to-date murine models form the basis for the majority of current knowledge. Pregnancy potentiates the acute lung injury after halogen exposure. In addition, halogen exposure precipitates a preeclamptic-like syndrome in mice. This phenotype is characterized by systemic and pulmonary hypertension, endothelial dysfunction, decreased cardiac output, placental injury and fetal growth restriction. This constellation contributes to increased maternal and fetal mortality observed after bromine exposure. Angiogenic imbalance is noted with overexpression of the soluble fms-like tyrosine kinase-1 (sFlt-1) form of the vascular endothelial growth factor receptor 1 reminiscent of human preeclampsia. Additional research is needed to further explore the effect of halogen gas exposure in pregnancy and to develop therapeutic interventions to mitigate risk to this unique population.
Keywords: Halogen; bromine; cardiopulmonary injury; chlorine; pregnancy.
Figures



References
-
- Addis DR, Lambert JA, Ren C, Doran S, Aggarwal S, Jilling T, Matalon S. 2020. Vascular Endothelial Growth Factor-121 Administration Mitigates Halogen Inhalation-Induced Pulmonary Injury and Fetal Growth Restriction in Pregnant Mice. Journal of the American Heart Association. 9(3):e013238. - PMC - PubMed
-
- Ahmad S, Ahmad A, Hendry-Hofer TB, Loader JE, Claycomb WC, Mozziconacci O, Schoneich C, Reisdorph N, Powell RL, Chandler JD et al. 2015. Sarcoendoplasmic reticulum Ca(2+) ATPase. A critical target in chlorine inhalation-induced cardiotoxicity. Am J Respir Cell Mol Biol. 52(4):492–502. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
