Germline BRCA, chemotherapy response scores, and survival in the neoadjuvant treatment of ovarian cancer
- PMID: 32131779
- PMCID: PMC7057666
- DOI: 10.1186/s12885-020-6688-8
Germline BRCA, chemotherapy response scores, and survival in the neoadjuvant treatment of ovarian cancer
Abstract
Background: To analyze the effects of BRCA1/2 mutations on chemotherapy response scores (CRS) and survival in a cohort of patients with advanced-stage ovarian cancer who were treated with neoadjuvant chemotherapy (NAC) followed by interval debulking surgery (IDS).
Methods: We retrospectively reviewed the medical records of 169 high-grade serous ovarian cancer patients who underwent a germline BRCA1/2 test and received three cycles of NAC at the Yonsei Cancer Center from 2006 to 2018. Chemotherapy response scores were compared in patients with and without BRCA1/2 mutations. The effects of BRCA1/2 mutations and CRS on survival were evaluated.
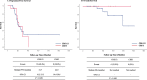
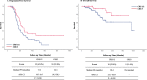
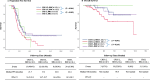
Results: BRCA1/2 mutations were detected in 47 (28.1%) of the 169 patients. Overall, 16 (34.0%) patients with BRCA1/2 mutations had a CRS 3 to chemotherapy compared to scores of 43 in patients (35.2%) without a mutation. Response scores of 3 in patients with BRCA1/2 mutations were not significantly associated with either improved progression-free survival (PFS) (P = 0.949) or overall survival (OS) (P = 0.168). However, CRS 3 in patients without BRCA mutations was significantly associated with both improved PFS (P = 0.030) and OS (P = 0.039). In patients with CRS1/2, carriers of BRCA1/2 mutations had better PFS (P = 0.0344) and OS (P = 0.043) than wild-type BRCA genotype patients.
Conclusion: In ovarian cancer patients treated with NAC, CRS did not predict survival for BRCA 1/2 mutation carriers but did for BRCA wild-type patients.
Keywords: Chemotherapy response scores; Germline BRCA; Neoadjuvant chemotherapy; Ovarian cancer.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

References
-
- Onda T, Matsumoto K, Shibata T, Sato A, Fukuda H, Konishi I, Kamura T, Yoshikawa H. Phase III trial of upfront debulking surgery versus neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers: Japan clinical oncology group study JCOG0602. Jpn J Clin Oncol. 2008;38(1):74–77. doi: 10.1093/jjco/hym145. - DOI - PubMed
-
- Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, Luesley D, Perren T, Bannoo S, Mascarenhas M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386(9990):249–257. doi: 10.1016/S0140-6736(14)62223-6. - DOI - PubMed
-
- Fagotti A, Ferrandina G, Vizzielli G, Fanfani F, Gallotta V, Chiantera V, Costantini B, Margariti PA, Gueli Alletti S, Cosentino F, et al. Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): final analysis of peri-operative outcome. Eur J Cancer. 2016;59:22–33. doi: 10.1016/j.ejca.2016.01.017. - DOI - PubMed
-
- Bohm S, Faruqi A, Said I, Lockley M, Brockbank E, Jeyarajah A, Fitzpatrick A, Ennis D, Dowe T, Santos JL, et al. Chemotherapy response score: development and validation of a system to quantify Histopathologic response to Neoadjuvant chemotherapy in Tubo-ovarian high-grade serous carcinoma. J Clin Oncol. 2015;33(22):2457–2463. doi: 10.1200/JCO.2014.60.5212. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

