Efficacy, immunogenicity, and safety of IC43 recombinant Pseudomonas aeruginosa vaccine in mechanically ventilated intensive care patients-a randomized clinical trial
- PMID: 32131866
- PMCID: PMC7057595
- DOI: 10.1186/s13054-020-2792-z
Efficacy, immunogenicity, and safety of IC43 recombinant Pseudomonas aeruginosa vaccine in mechanically ventilated intensive care patients-a randomized clinical trial
Abstract
Background: Pseudomonas aeruginosa infections are a serious threat in intensive care units (ICUs). The aim of this confirmatory, randomized, multicenter, placebo-controlled, double-blind, phase 2/3 study was to assess the efficacy, immunogenicity, and safety of IC43 recombinant Pseudomonas aeruginosa vaccine in non-surgical ICU patients.
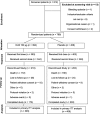
Methods: Eight hundred patients aged 18 to 80 years admitted to the ICU with expected need for mechanical ventilation for ≥ 48 h were randomized 1:1 to either IC43 100 μg or saline placebo, given in two vaccinations 7 days apart. The primary efficacy endpoint was all-cause mortality in patients 28 days after the first vaccination. Immunogenicity and safety were also evaluated.
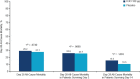
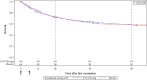
Findings: All-cause mortality rates at day 28 were 29.2% vs 27.7% in the IC43 and placebo groups, respectively (P = .67). Overall survival (Kaplan-Meier survival estimates, P = .46) and proportion of patients with ≥ one confirmed P. aeruginosa invasive infection or respiratory tract infection also did not differ significantly between both groups. The geometric mean fold increase in OprF/I titers was 1.5 after the first vaccination, 20 at day 28, after the second vaccination, and 2.9 at day 180. Significantly more patients in the placebo group (96.5%) had ≥ one adverse event (AE) versus the IC43 100 μg group (93.1%) (P = .04). The most frequently reported severe AEs in the IC43 and placebo groups were respiratory failure (6.9% vs 5.7%, respectively), septic shock (4.1% vs 6.5%), cardiac arrest (4.3% vs 5.7%), multiorgan failure (4.6% vs 5.5%), and sepsis (4.6% vs 4.2%). No related serious AEs were reported in the IC43 group.
Interpretation: The IC43 100 μg vaccine was well tolerated in this large population of medically ill, mechanically ventilated patients. The vaccine achieved high immunogenicity but provided no clinical benefit over placebo in terms of overall mortality.
Trial registration: https://clinicaltrials.gov (NCT01563263). Registration was sent to ClinicalTrials.gov on March 14, 2012, but posted by ClinicalTrials.gov on March 26, 2012. The first subject was included in the trial on March 22, 2012.
Keywords: Intensive care; Mechanical ventilation; Pseudomonas aeruginosa; Vaccination.
Conflict of interest statement
SE, KD, and NW are Valneva employees and own stock and share options in Valneva. NK was an employee of GSK during the study and now serves as a consultant to Valneva. JR and CA have received honorary as consultants for the sponsor. CA, RW, PD, HS, JAL, TS, JC, CZ, AMH, PE, MVL, ZM, IV, BS, MH, VŠ, HS and JR received research funding for the trial from the sponsor.
Figures
References
-
- Ewans T. Prevention and control of nosocomial infection in the intensive care unit. 4. New York: Lippincot-Ravan; Irwin and Rippe’s intensive care medicine - NLM Catalog - NCBI; 1999.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

