MEF2c-Dependent Downregulation of Myocilin Mediates Cancer-Induced Muscle Wasting and Associates with Cachexia in Patients with Cancer
- PMID: 32132110
- PMCID: PMC7250164
- DOI: 10.1158/0008-5472.CAN-19-1558
MEF2c-Dependent Downregulation of Myocilin Mediates Cancer-Induced Muscle Wasting and Associates with Cachexia in Patients with Cancer
Abstract
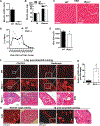
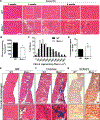
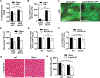
Skeletal muscle wasting is a devastating consequence of cancer that contributes to increased complications and poor survival, but is not well understood at the molecular level. Herein, we investigated the role of Myocilin (Myoc), a skeletal muscle hypertrophy-promoting protein that we showed is downregulated in multiple mouse models of cancer cachexia. Loss of Myoc alone was sufficient to induce phenotypes identified in mouse models of cancer cachexia, including muscle fiber atrophy, sarcolemmal fragility, and impaired muscle regeneration. By 18 months of age, mice deficient in Myoc showed significant skeletal muscle remodeling, characterized by increased fat and collagen deposition compared with wild-type mice, thus also supporting Myoc as a regulator of muscle quality. In cancer cachexia models, maintaining skeletal muscle expression of Myoc significantly attenuated muscle loss, while mice lacking Myoc showed enhanced muscle wasting. Furthermore, we identified the myocyte enhancer factor 2 C (MEF2C) transcription factor as a key upstream activator of Myoc whose gain of function significantly deterred cancer-induced muscle wasting and dysfunction in a preclinical model of pancreatic ductal adenocarcinoma (PDAC). Finally, compared with noncancer control patients, MYOC was significantly reduced in skeletal muscle of patients with PDAC defined as cachectic and correlated with MEF2c. These data therefore identify disruptions in MEF2c-dependent transcription of Myoc as a novel mechanism of cancer-associated muscle wasting that is similarly disrupted in muscle of patients with cachectic cancer. SIGNIFICANCE: This work identifies a novel transcriptional mechanism that mediates skeletal muscle wasting in murine models of cancer cachexia that is disrupted in skeletal muscle of patients with cancer exhibiting cachexia.
©2020 American Association for Cancer Research.
Conflict of interest statement
Figures
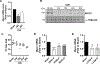

Similar articles
-
Complement pathway activation mediates pancreatic cancer-induced muscle wasting and pathological remodeling.J Clin Invest. 2025 Apr 8;135(12):e178806. doi: 10.1172/JCI178806. eCollection 2025 Jun 16. J Clin Invest. 2025. PMID: 40198138 Free PMC article.
-
FoxP1 is a transcriptional repressor associated with cancer cachexia that induces skeletal muscle wasting and weakness.J Cachexia Sarcopenia Muscle. 2021 Apr;12(2):421-442. doi: 10.1002/jcsm.12666. Epub 2021 Feb 1. J Cachexia Sarcopenia Muscle. 2021. PMID: 33527776 Free PMC article.
-
Disruption of MEF2C signaling and loss of sarcomeric and mitochondrial integrity in cancer-induced skeletal muscle wasting.Aging (Albany NY). 2012 Feb;4(2):133-43. doi: 10.18632/aging.100436. Aging (Albany NY). 2012. PMID: 22361433 Free PMC article.
-
Skeletal muscle mass is controlled by the MRF4-MEF2 axis.Curr Opin Clin Nutr Metab Care. 2018 May;21(3):164-167. doi: 10.1097/MCO.0000000000000456. Curr Opin Clin Nutr Metab Care. 2018. PMID: 29389722 Review.
-
Mechanisms to explain wasting of muscle and fat in cancer cachexia.Curr Opin Support Palliat Care. 2007 Dec;1(4):293-8. doi: 10.1097/SPC.0b013e3282f34738. Curr Opin Support Palliat Care. 2007. PMID: 18685378 Review.
Cited by
-
Molecular Mechanisms of Skeletal Muscle Hypertrophy.J Neuromuscul Dis. 2021;8(2):169-183. doi: 10.3233/JND-200568. J Neuromuscul Dis. 2021. PMID: 33216041 Free PMC article. Review.
-
Complement pathway activation mediates pancreatic cancer-induced muscle wasting and pathological remodeling.J Clin Invest. 2025 Apr 8;135(12):e178806. doi: 10.1172/JCI178806. eCollection 2025 Jun 16. J Clin Invest. 2025. PMID: 40198138 Free PMC article.
-
Comparative transcriptome analysis of longissimus dorsi muscle reveal potential genes affecting meat trait in Chinese indigenous Xiang pig.Sci Rep. 2024 Apr 11;14(1):8486. doi: 10.1038/s41598-024-58971-2. Sci Rep. 2024. PMID: 38605105 Free PMC article.
-
Blocking muscle wasting via deletion of the muscle-specific E3 ubiquitin ligase MuRF1 impedes pancreatic tumor growth.Res Sq [Preprint]. 2023 Feb 9:rs.3.rs-2524562. doi: 10.21203/rs.3.rs-2524562/v1. Res Sq. 2023. Update in: Commun Biol. 2023 May 13;6(1):519. doi: 10.1038/s42003-023-04902-2. PMID: 36798266 Free PMC article. Updated. Preprint.
-
Determining the Potential Roles of Branched-Chain Amino Acids in the Regulation of Muscle Growth in Common Carp (Cyprinus carpio) Based on Transcriptome and MicroRNA Sequencing.Aquac Nutr. 2023 Jun 3;2023:7965735. doi: 10.1155/2023/7965735. eCollection 2023. Aquac Nutr. 2023. PMID: 37303609 Free PMC article.
References
-
- Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Primers 2018;4:17105. - PubMed
-
- Kazemi-Bajestani SM, Mazurak VC, Baracos V. Computed tomography-defined muscle and fat wasting are associated with cancer clinical outcomes. Semin Cell Dev Biol 2016;54:2–10 - PubMed
-
- Mendes MC, Pimentel GD, Costa FO, Carvalheira JB. Molecular and neuroendocrine mechanisms of cancer cachexia. J Endocrinol 2015;226:R29–43 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

