How Plants Sense and Respond to Stressful Environments
- PMID: 32132112
- PMCID: PMC7140927
- DOI: 10.1104/pp.19.01464
How Plants Sense and Respond to Stressful Environments
Abstract
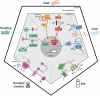
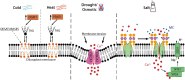
Plants are exposed to an ever-changing environment to which they have to adjust accordingly. Their response is tightly regulated by complex signaling pathways that all start with stimulus perception. Here, we give an overview of the latest developments in the perception of various abiotic stresses, including drought, salinity, flooding, and temperature stress. We discuss whether proposed perception mechanisms are true sensors, which is well established for some abiotic factors but not yet fully elucidated for others. In addition, we review the downstream cellular responses, many of which are shared by various stresses but result in stress-specific physiological and developmental output. New sensing mechanisms have been identified, including heat sensing by the photoreceptor phytochrome B, salt sensing by glycosylinositol phosphorylceramide sphingolipids, and drought sensing by the specific calcium influx channel OSCA1. The simultaneous occurrence of multiple stress conditions shows characteristic downstream signaling signatures that were previously considered general signaling responses. The integration of sensing of multiple stress conditions and subsequent signaling responses is a promising venue for future research to improve the understanding of plant abiotic stress perception.
© 2020 American Society of Plant Biologists. All Rights Reserved.
Figures


References
-
- Alvarez S, Roy Choudhury S, Hicks LM, Pandey S(2013) Quantitative proteomics-based analysis supports a significant role of GTG proteins in regulation of ABA response in Arabidopsis roots. J Proteome Res 12: 1487–1501 - PubMed
-
- Al-Whaibi MH.(2011) Plant heat-shock proteins: A mini review. J King Saud Univ Sci 23: 139–150
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

