The Women's Health Initiative Estrogen-alone Trial had differential disease and medical expenditure consequences across age groups
- PMID: 32132440
- PMCID: PMC7255959
- DOI: 10.1097/GME.0000000000001517
The Women's Health Initiative Estrogen-alone Trial had differential disease and medical expenditure consequences across age groups
Abstract
Objectives: The Women's Health Initiative (WHI) randomized trial identified age differences in the benefit-risk profile of estrogen-alone (ET) use. The impact of WHI trial on disease-associated medical expenditures attributable to subsequent decreased ET utilization has, however, not been measured. Therefore, the objective of this analysis was to quantify the age-specific disease-associated medical expenditures attributable to reduced ET utilization after the WHI Hormone Therapy (HT) trials.
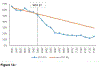
Methods: Population-level disease counts and associated expenditures between 2003 and 2015 were compared between an observed ET-user population versus a hypothetical ET-user population assuming absence of the WHI HT trials, constructed by extrapolating ET utilization rates from 1996 to 2002 assuming pre-WHI HT rates would have continued without publication of the WHI HT trial data (2002-2004). Analyses were stratified by age (50-59, 60-69, and 70-79 years). Input data were extracted from Medical Expenditure Panel Survey and the literature. The primary outcomes were: ET utilization, chronic diseases (breast cancer, stroke, coronary heart disease, colorectal cancer, pulmonary embolism, and hip fracture) and disease-associated direct medical expenditures.
Results: Over 13 years, the decline in ET utilization was associated with $4.1 billion expenditure for excess chronic diseases (37,549 excess events) among women in their 50s, compared to savings of $1.5 billion and $4.4 billion for diseases averted by lower ET utilization among women in their 60s (13,495 fewer events) and 70s (40,792 fewer events), respectively.
Conclusion: The decline in ET utilization had differential disease and expenditure consequences by age groups in the United States. These results are limited by the lack of inclusion of vasomotor symptom benefit and costs of alternative medications for these symptoms in the analysis.
Conflict of interest statement
Conflict of interest/financial disclosure: Dr. Chlebowski has received funding from Pfizer. Dr. Roth has received funding from Bayer. The other authors have no relevant disclosures.
Figures


Comment in
-
Adding up the healthcare costs when estrogen therapy is avoided after hysterectomy.Menopause. 2020 Jun;27(6):625-627. doi: 10.1097/GME.0000000000001548. Menopause. 2020. PMID: 32217889 No abstract available.
References
-
- Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–12. - PubMed
-
- The NHTPSAP. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–53. - PubMed
-
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

