Alcohol-derived DNA crosslinks are repaired by two distinct mechanisms
- PMID: 32132710
- PMCID: PMC7116288
- DOI: 10.1038/s41586-020-2059-5
Alcohol-derived DNA crosslinks are repaired by two distinct mechanisms
Abstract
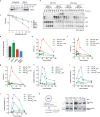
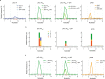
Acetaldehyde is a highly reactive, DNA-damaging metabolite that is produced upon alcohol consumption1. Impaired detoxification of acetaldehyde is common in the Asian population, and is associated with alcohol-related cancers1,2. Cells are protected against acetaldehyde-induced damage by DNA crosslink repair, which when impaired causes Fanconi anaemia (FA), a disease resulting in failure to produce blood cells and a predisposition to cancer3,4. The combined inactivation of acetaldehyde detoxification and the FA pathway induces mutation, accelerates malignancies and causes the rapid attrition of blood stem cells5-7. However, the nature of the DNA damage induced by acetaldehyde and how this is repaired remains a key question. Here we generate acetaldehyde-induced DNA interstrand crosslinks and determine their repair mechanism in Xenopus egg extracts. We find that two replication-coupled pathways repair these lesions. The first is the FA pathway, which operates using excision-analogous to the mechanism used to repair the interstrand crosslinks caused by the chemotherapeutic agent cisplatin. However, the repair of acetaldehyde-induced crosslinks results in increased mutation frequency and an altered mutational spectrum compared with the repair of cisplatin-induced crosslinks. The second repair mechanism requires replication fork convergence, but does not involve DNA incisions-instead the acetaldehyde crosslink itself is broken. The Y-family DNA polymerase REV1 completes repair of the crosslink, culminating in a distinct mutational spectrum. These results define the repair pathways of DNA interstrand crosslinks caused by an endogenous and alcohol-derived metabolite, and identify an excision-independent mechanism.
Conflict of interest statement
Authors declare no competing interests.
Figures









Comment in
-
A safe fix for alcohol-derived DNA damage.Nature. 2020 Mar;579(7800):499-500. doi: 10.1038/d41586-020-00462-1. Nature. 2020. PMID: 32210381 No abstract available.
-
A new varietal of DNA interstrand crosslink repair.Cell Res. 2020 Jun;30(6):459-460. doi: 10.1038/s41422-020-0321-x. Cell Res. 2020. PMID: 32346072 Free PMC article. No abstract available.
References
-
- Lai CL, et al. Dominance of the Inactive Asian Variant Over Activity and Protein Contents of Mitochondrial Aldehyde Dehydrogenase 2 in Human Liver. Alcoholism-Clinical and Experimental Research. 2014;38:44–50. - PubMed
-
- Langevin F, Crossan GP, Rosado IV, Arends MJ, Patel KJ. Fancd2 counteracts the toxic effects of naturally produced aldehydes in mice. Nature. 2011;475:53–8. - PubMed
-
- Garaycoechea JI, Patel KJ. Why does the bone marrow fail in Fanconi anemia? Blood. 2014;123:26–34. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

