Decoy exosomes provide protection against bacterial toxins
- PMID: 32132711
- PMCID: PMC7519780
- DOI: 10.1038/s41586-020-2066-6
Decoy exosomes provide protection against bacterial toxins
Abstract
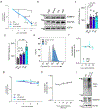
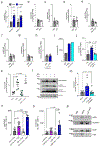
The production of pore-forming toxins that disrupt the plasma membrane of host cells is a common virulence strategy for bacterial pathogens such as methicillin-resistant Staphylococcus aureus (MRSA)1-3. It is unclear, however, whether host species possess innate immune mechanisms that can neutralize pore-forming toxins during infection. We previously showed that the autophagy protein ATG16L1 is necessary for protection against MRSA strains encoding α-toxin4-a pore-forming toxin that binds the metalloprotease ADAM10 on the surface of a broad range of target cells and tissues2,5,6. Autophagy typically involves the targeting of cytosolic material to the lysosome for degradation. Here we demonstrate that ATG16L1 and other ATG proteins mediate protection against α-toxin through the release of ADAM10 on exosomes-extracellular vesicles of endosomal origin. Bacterial DNA and CpG DNA induce the secretion of ADAM10-bearing exosomes from human cells as well as in mice. Transferred exosomes protect host cells in vitro by serving as scavengers that can bind multiple toxins, and improve the survival of mice infected with MRSA in vivo. These findings indicate that ATG proteins mediate a previously unknown form of defence in response to infection, facilitating the release of exosomes that serve as decoys for bacterially produced toxins.
Figures

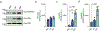
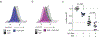

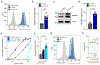
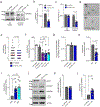
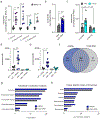

Comment in
-
Distracting your enemy with bubbles.Nat Rev Microbiol. 2020 May;18(5):263. doi: 10.1038/s41579-020-0355-6. Nat Rev Microbiol. 2020. PMID: 32203295 No abstract available.
-
Exosomes as Sentinels against Bacterial Pathogens.Dev Cell. 2020 Apr 20;53(2):138-139. doi: 10.1016/j.devcel.2020.03.022. Dev Cell. 2020. PMID: 32315610 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- UL1 TR001445/TR/NCATS NIH HHS/United States
- P30 CA016087/CA/NCI NIH HHS/United States
- R01 DK093668/DK/NIDDK NIH HHS/United States
- P01 CA023766/CA/NCI NIH HHS/United States
- R01 AI130945/AI/NIAID NIH HHS/United States
- F31 HL137304/HL/NHLBI NIH HHS/United States
- R01 AI105129/AI/NIAID NIH HHS/United States
- R01 DK103788/DK/NIDDK NIH HHS/United States
- R01 HL123340/HL/NHLBI NIH HHS/United States
- R01 AI099394/AI/NIAID NIH HHS/United States
- R01 HL125816/HL/NHLBI NIH HHS/United States
- T32 AI007180/AI/NIAID NIH HHS/United States
- R01 AI121244/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

