Olfactory receptor and circuit evolution promote host specialization
- PMID: 32132713
- PMCID: PMC7100913
- DOI: 10.1038/s41586-020-2073-7
Olfactory receptor and circuit evolution promote host specialization
Abstract
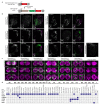
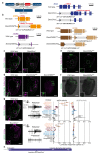
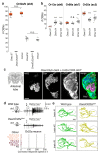
The evolution of animal behaviour is poorly understood1,2. Despite numerous correlations between interspecific divergence in behaviour and nervous system structure and function, demonstrations of the genetic basis of these behavioural differences remain rare3-5. Here we develop a neurogenetic model, Drosophila sechellia, a species that displays marked differences in behaviour compared to its close cousin Drosophila melanogaster6,7, which are linked to its extreme specialization on noni fruit (Morinda citrifolia)8-16. Using calcium imaging, we identify olfactory pathways in D. sechellia that detect volatiles emitted by the noni host. Our mutational analysis indicates roles for different olfactory receptors in long- and short-range attraction to noni, and our cross-species allele-transfer experiments demonstrate that the tuning of one of these receptors is important for species-specific host-seeking. We identify the molecular determinants of this functional change, and characterize their evolutionary origin and behavioural importance. We perform circuit tracing in the D. sechellia brain, and find that receptor adaptations are accompanied by increased sensory pooling onto interneurons as well as species-specific central projection patterns. This work reveals an accumulation of molecular, physiological and anatomical traits that are linked to behavioural divergence between species, and defines a model for investigating speciation and the evolution of the nervous system.
Conflict of interest statement
The authors declare no competing interests.
Figures








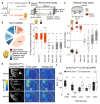


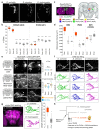
Comment in
-
How a fly came to love the vomit fruit.Nature. 2020 Mar;579(7799):345-346. doi: 10.1038/d41586-020-00535-1. Nature. 2020. PMID: 32173720 No abstract available.
-
Sensory Evolution: Making Sense of the Noni Scent.Curr Biol. 2020 Jun 22;30(12):R712-R715. doi: 10.1016/j.cub.2020.04.059. Curr Biol. 2020. PMID: 32574635
References
-
- Bendesky A, Bargmann CI. Genetic contributions to behavioural diversity at the gene-environment interface. Nat Rev Genet. 2011;12:809–820. - PubMed
-
- Ding Y, Berrocal A, Morita T, Longden KD, Stern DL. Natural courtship song variation caused by an intronic retroelement in an ion channel gene. Nature. 2016;536:329–332. - PubMed
-
- Weber JN, Peterson BK, Hoekstra HE. Discrete genetic modules are responsible for complex burrow evolution in Peromyscus mice. Nature. 2013;493:402–405. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

