C-Type Lectin-Like Receptors: Head or Tail in Cell Death Immunity
- PMID: 32133013
- PMCID: PMC7040094
- DOI: 10.3389/fimmu.2020.00251
C-Type Lectin-Like Receptors: Head or Tail in Cell Death Immunity
Abstract
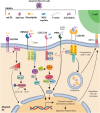
C-type lectin-like receptors (CLRs) represent a family of transmembrane pattern recognition receptors, expressed primarily by myeloid cells. They recognize not only pathogen moieties for host defense, but also modified self-antigens such as damage-associated molecular patterns released from dead cells. Upon ligation, CLR signaling leads to the production of inflammatory mediators to shape amplitude, duration and outcome of the immune response. Thus, following excessive injury, dysregulation of these receptors leads to the development of inflammatory diseases. Herein, we will focus on four CLRs of the "Dectin family," shown to decode the immunogenicity of cell death. CLEC9A on dendritic cells links F-actin exposed by dying cells to favor cross-presentation of dead-cell associated antigens to CD8+ T cells. Nevertheless, CLEC9A exerts also feedback mechanisms to temper neutrophil recruitment and prevent additional tissue damage. MINCLE expressed by macrophages binds nuclear SAP130 released by necrotic cells to potentiate pro-inflammatory responses. However, the consequent inflammation can exacerbate pathogenesis of inflammatory diseases. Moreover, in a tumor microenvironment, MINCLE induces macrophage-induced immune suppression and cancer progression. Similarly, triggering of LOX-1 by oxidized LDL, amplifies pro-inflammatory response but promotes tumor immune escape and metastasis. Finally, CLEC12A that recognizes monosodium urate crystals formed during cell death, inhibits activating signals to prevent detrimental inflammation. Interestingly, CLEC12A also sustains type-I IFN response to finely tune immune responses in case of viral-induced collateral damage. Therefore, CLRs acting in concert as sensors of injury, could be used in a targeted way to treat numerous diseases such as allergies, obesity, tumors, and autoimmunity.
Keywords: C-type lectin-like receptors; cross-presentation; dead cells; sterile inflammation; tissue injury.
Copyright © 2020 Drouin, Saenz and Chiffoleau.
Figures
References
-
- Lockshin RA, Williams CM. Programmed cell death-II. endocrine potentiation of the breakdown of the intersegmental muscles of silkmoths. J Insect Physiol. (1964) 10:643–9. 10.1016/0022-1910(64)90034-4 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

