Immunization against Leishmania major infection in BALB/c mice using a subunit-based DNA vaccine derived from TSA, LmSTI1, KMP11, and LACK predominant antigens
- PMID: 32133069
- PMCID: PMC7043880
- DOI: 10.22038/IJBMS.2019.14051
Immunization against Leishmania major infection in BALB/c mice using a subunit-based DNA vaccine derived from TSA, LmSTI1, KMP11, and LACK predominant antigens
Abstract
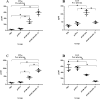
Objectives: To design a multivalent DNA vaccine encoding the most immunogenic regions of the Leishmania major antigens including TSA (Thiol-specific antioxidant protein), LmSTI1 (Leishmania major stress-inducible protein1), LACK (Leishmania homologue of receptors for activated C Kinase), and KMP11 (kinetoplastid membrane protein-11) on BALB/c mice.
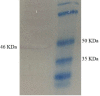
Materials and methods: The chimeric construct was generated including the most immunogenic epitopes containing a combination of domains and oligopeptides of the aforementioned genes. The construct was cloned into pcDNA 3.1 plasmid and named "pleish-dom." Following intramuscular injection of mice, the capability of the vector pleish-dom alone and with pIL-12 (expressing murine IL-12) to raise protective cytokines and parasite burden was evaluated in the BALB/c mice as a susceptible animal model against L. major.
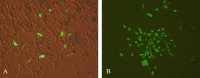
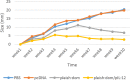
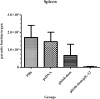
Results: The immunized mice with pleish-dom/pIL-12 showed the highest and the lowest levels of interferon-gamma (IFN-γ) and interleukin-10 (IL-10), as well as the lowest leishmanin skin test (LST) reactions, were found through 8 weeks post-infection.
Conclusion: Although the obtained DNA vaccine from the immunogenic chimeric protein of L. major antigens was able to induce a high level of IFN-γ, it partially protected mice against L. major. However co-administration with pIL-12 led to shift immune response to Th1 phenotype, granuloma formation, and lowered parasite burden, and consequently distinct protection was found.
Keywords: BALB/c mice; Cytokines; DNA; Epitopes; Leishmania major; Vaccines.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



References
-
- O mondiale de la Santé, World Health Organization. Leishmaniasis in high-burden countries: an epidemiological update based on data reported in 2014. Wkly Epidemiol Rec. 2016;91:287–296. - PubMed
-
- Leishmaniases WECotCot, Meeting WECotCotL, World Health Organization. Control of the Leishmaniases: Report of a WHO Expert Committee. World Health Organization . 1990 - PubMed
-
- Khanjani N, González U, Leonardi-Bee J, Mohebali M, Saffari M, Khamesipour A. Vaccines for preventing cutaneous leishmaniasis. Cochrane Database Syst Rev . 2009
-
- Nadim A, Javadian E, Mohebali M. The experience of leishmanization in the Islamic Republic of Iran. East Mediterr Health J. 1997;3:284–290.
-
- Mohebali M, Khamesipour A, Mobedi I, Zarei Z, Hashemi-Fesharki R. Double-blind randomized efficacy field trial of alum precipitated autoclaved Leishmania major vaccine mixed with BCG against canine visceral leishmaniasis in Meshkin-Shahr district, IR Iran. Vaccine. 2004;22:4097–4100. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
