Early real-world effectiveness of ustekinumab for Crohn's disease
- PMID: 32133109
- PMCID: PMC7043072
- DOI: 10.1136/flgastro-2019-101237
Early real-world effectiveness of ustekinumab for Crohn's disease
Abstract
Objective: To understand the effectiveness of ustekinumab in treating Crohn's disease (CD) in a UK real-world setting.
Design: Retrospective cohort study using prospectively maintained clinical records.
Setting: Single UK inflammatory bowel disease centre.
Patients: Adult patients with an established diagnosis of CD prescribed ustekinumab outside of clinical trials at University Hospital Southampton (UHS).
Interventions: Ustekinumab, a monoclonal antibody to the shared p40 subunit of interleukin (IL) 12 and IL-23 as part of routine clinical care.
Main outcome measures: Effectiveness as measured by an improvement in physician's global assessment, drug persistence and improvement in biomarkers (C-reactive protein (CRP), albumin and calprotectin).
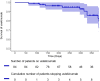
Results: 84 patients were included, 72 had a postinduction review and 49 had 1-year data. At postinduction clinical review, clinical response occurred in 53% of patients and clinical remission occurred in 8%. For patients on ustekinumab at 1 year, clinical response occurred in 71% and remission in 14%. Adverse events included four patients with infections requiring admission, one drug-related rash, five CD surgeries and two CD exacerbations.
Conclusions: Ustekinumab was well tolerated in a complex UK CD population and demonstrated benefit to patients in terms of clinical response and improvement of biomarkers and with some patients attaining clinical remission. No unexpected safety signals were seen.
Keywords: crohn's disease; ibd clinical; inflammatory bowel disease.
© Author(s) (or their employer(s)) 2020. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: Outside the submitted work the following interests are declared. RJH: personal fees from Abbvie & Janssen; non-financial support from Falk. MM: non-financial support from Falk, MSD, Janssen & Takeda. MB: Personal fees from Abbvie, Falk, Hospira, Janssen, MSD, Napp, Takeda & Pfizer. MG: personal fees and non-financial support from Abbvie & MSD; personal fees from Takeda; non-financial support from Falk & Janssen. JRFC: grants and personal fees from Samsung, Pfizer & Biogen; personal fees and non-financial support from Janssen & Abbvie; grants, personal fees and non-financial support from Takeda; personal fees from MSD, Sandoz, Celltrion & NAPP.
Figures



References
-
- NICE Vedolizumab for treating moderately to severely active Crohn’s disease after prior therapy | Guidance and guidelines, 2015. Available: https://www.nice.org.uk/guidance/ta352/chapter/1-Guidance [Accessed 01 May 2019].
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
