Melatonin alleviates intervertebral disc degeneration by disrupting the IL-1β/NF-κB-NLRP3 inflammasome positive feedback loop
- PMID: 32133213
- PMCID: PMC7028926
- DOI: 10.1038/s41413-020-0087-2
Melatonin alleviates intervertebral disc degeneration by disrupting the IL-1β/NF-κB-NLRP3 inflammasome positive feedback loop
Abstract
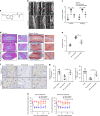
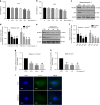
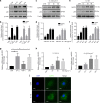
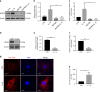
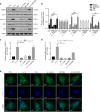
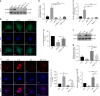
The inflammatory response is induced by the overexpression of inflammatory cytokines, mainly interleukin (IL)-1β, and is one of the main causes of intervertebral disc degeneration (IVDD). NLR pyrin domain containing 3 (NLRP3) inflammasome activation is an important source of IL-1β. As an anti-inflammatory neuroendocrine hormone, melatonin plays various roles in different pathophysiological conditions. However, its roles in IVDD are still not well understood and require more examination. First, we demonstrated that melatonin delayed the progression of IVDD and relieved IVDD-related low back pain in a rat needle puncture IVDD model; moreover, NLRP3 inflammasome activation (NLRP3, p20, and IL-1β levels) was significantly upregulated in severely degenerated human discs and a rat IVDD model. Subsequently, an IL-1β/NF-κB-NLRP3 inflammasome activation positive feedback loop was found in nucleus pulposus (NP) cells that were treated with IL-1β. In these cells, expression of NLRP3 and p20 was significantly increased, NF-κB signaling was involved in this regulation, and mitochondrial reactive oxygen species (mtROS) production increased. Furthermore, we found that melatonin disrupted the IL-1β/NF-κB-NLRP3 inflammasome activation positive feedback loop in vitro and in vivo. Melatonin treatment decreased NLRP3, p20, and IL-1β levels by inhibiting NF-κB signaling and downregulating mtROS production. Finally, we showed that melatonin mediated the disruption of the positive feedback loop of IL-1β in vivo. In this study, we showed for the first time that IL-1β promotes its own expression by upregulating NLRP3 inflammasome activation. Furthermore, melatonin disrupts the IL-1β positive feedback loop and may be a potential therapeutic agent for IVDD.
Keywords: Metabolism; Neurophysiology.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures


Comment in
-
Targeting IL-1β expression in IVDD.Nat Rev Rheumatol. 2020 Apr;16(4):188. doi: 10.1038/s41584-020-0403-7. Nat Rev Rheumatol. 2020. PMID: 32144404 No abstract available.
References
-
- Vlaeyen, J. W. S. et al. Low back pain. Nat. Rev. Dis.Primers4, 52 (2018). - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

