FGF Signaling Pathway: A Key Regulator of Stem Cell Pluripotency
- PMID: 32133359
- PMCID: PMC7040165
- DOI: 10.3389/fcell.2020.00079
FGF Signaling Pathway: A Key Regulator of Stem Cell Pluripotency
Abstract
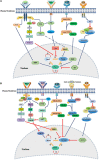
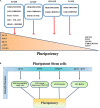
Pluripotent stem cells (PSCs) isolated in vitro from embryonic stem cells (ESCs), induced PSC (iPSC) and also post-implantation epiblast-derived stem cells (EpiSCs) are known for their two unique characteristics: the ability to give rise to all somatic lineages and the self-renewal capacity. Numerous intrinsic signaling pathways contribute to the maintenance of the pluripotency state of stem cells by tightly controlling key transcriptional regulators of stemness including sex determining region Y box 2 (Sox-2), octamer-binding transcription factor (Oct)3/4, krueppel-like factor 4 (Klf-4), Nanog, and c-Myc. Signaling by fibroblast growth factor (FGF) is of critical importance in regulating stem cells pluripotency. The FGF family is comprised of 22 ligands that interact with four FGF receptors (FGFRs). FGF/FGFR signaling governs fundamental cellular processes such as cell survival, proliferation, migration, differentiation, embryonic development, organogenesis, tissue repair/regeneration, and metabolism. FGF signaling is mediated by the activation of RAS - mitogen-activated protein kinase (MAPK), phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)-AKT, Phospholipase C Gamma (PLCγ), and signal transducers and activators of transcription (STAT), which intersects and synergizes with other signaling pathways such as Wnt, retinoic acid (RA) and transforming growth factor (TGF)-β signaling. In the current review, we summarize the role of FGF signaling in the maintenance of pluripotency state of stem cells through regulation of key transcriptional factors.
Keywords: FGF; pluripotency; self-renewal; stem cells; transcription factor.
Copyright © 2020 Mossahebi-Mohammadi, Quan, Zhang and Li.
Figures
Similar articles
-
The Role of Fibroblast Growth Factor (FGF) Signaling in Tissue Repair and Regeneration.Cells. 2021 Nov 19;10(11):3242. doi: 10.3390/cells10113242. Cells. 2021. PMID: 34831463 Free PMC article. Review.
-
Multiple coagulation factor deficiency protein 2 contains the ability to support stem cell self-renewal.FASEB J. 2013 Aug;27(8):3298-305. doi: 10.1096/fj.13-228825. Epub 2013 May 9. FASEB J. 2013. PMID: 23660967
-
CDK1-PDK1-PI3K/Akt signaling pathway regulates embryonic and induced pluripotency.Cell Death Differ. 2017 Jan;24(1):38-48. doi: 10.1038/cdd.2016.84. Epub 2016 Sep 16. Cell Death Differ. 2017. PMID: 27636107 Free PMC article.
-
Efficient derivation of bovine embryonic stem cells needs more than active core pluripotency factors.Mol Reprod Dev. 2012 Jul;79(7):461-77. doi: 10.1002/mrd.22051. Epub 2012 May 31. Mol Reprod Dev. 2012. PMID: 22573702
-
The Pleiotropic Effects of the Canonical Wnt Pathway in Early Development and Pluripotency.Genes (Basel). 2018 Feb 14;9(2):93. doi: 10.3390/genes9020093. Genes (Basel). 2018. PMID: 29443926 Free PMC article. Review.
Cited by
-
Mechanisms of Pharmaceutical Therapy and Drug Resistance in Esophageal Cancer.Front Cell Dev Biol. 2021 Feb 11;9:612451. doi: 10.3389/fcell.2021.612451. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33644048 Free PMC article. Review.
-
Role of glycosphingolipid SSEA-3 and FGF2 in the stemness and lineage commitment of multilineage differentiating stress enduring (MUSE) cells.Cell Prolif. 2023 Jan;56(1):e13345. doi: 10.1111/cpr.13345. Epub 2022 Oct 12. Cell Prolif. 2023. PMID: 36225120 Free PMC article.
-
KLF4 and CD55 expression and function depend on each other.Front Immunol. 2024 Feb 9;14:1290684. doi: 10.3389/fimmu.2023.1290684. eCollection 2023. Front Immunol. 2024. PMID: 38406578 Free PMC article.
-
Drug-Loaded Bioscaffolds for Osteochondral Regeneration.Pharmaceutics. 2024 Aug 21;16(8):1095. doi: 10.3390/pharmaceutics16081095. Pharmaceutics. 2024. PMID: 39204440 Free PMC article. Review.
-
Automated high-content imaging in iPSC-derived neuronal progenitors.SLAS Discov. 2023 Mar;28(2):42-51. doi: 10.1016/j.slasd.2022.12.002. Epub 2023 Jan 5. SLAS Discov. 2023. PMID: 36610640 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

