Mitochondrial ROS Formation in the Pathogenesis of Diabetic Cardiomyopathy
- PMID: 32133373
- PMCID: PMC7040199
- DOI: 10.3389/fcvm.2020.00012
Mitochondrial ROS Formation in the Pathogenesis of Diabetic Cardiomyopathy
Abstract
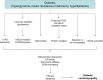
Diabetic cardiomyopathy is a result of diabetes-induced changes in the structure and function of the heart. Hyperglycemia affects multiple pathways in the diabetic heart, but excessive reactive oxygen species (ROS) generation and oxidative stress represent common denominators associated with adverse tissue remodeling. Indeed, key processes underlying cardiac remodeling in diabetes are redox sensitive, including inflammation, organelle dysfunction, alteration in ion homeostasis, cardiomyocyte hypertrophy, apoptosis, fibrosis, and contractile dysfunction. Extensive experimental evidence supports the involvement of mitochondrial ROS formation in the alterations characterizing the diabetic heart. In this review we will outline the central role of mitochondrial ROS and alterations in the redox status contributing to the development of diabetic cardiomyopathy. We will discuss the role of different sources of ROS involved in this process, with a specific emphasis on mitochondrial ROS producing enzymes within cardiomyocytes. Finally, the therapeutic potential of pharmacological inhibitors of ROS sources within the mitochondria will be discussed.
Keywords: diabetic cardiomyopathy; diabetic complication; mitochondria; oxidative stress; reactive oxygen species.
Copyright © 2020 Kaludercic and Di Lisa.
Figures

References
-
- Maack C, Lehrke M, Backs J, Heinzel FR, Hulot JS, Marx N, et al. Heart failure and diabetes: metabolic alterations and therapeutic interventions: a state-of-the-art review from the translational research committee of the heart failure association-european society of cardiology. Eur Heart J. (2018) 39:4243–54. 10.1093/eurheartj/ehy596 - DOI - PMC - PubMed
-
- Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. (2008) 26:77–82. 10.2337/diaclin.26.2.77 - DOI
Publication types
LinkOut - more resources
Full Text Sources

