Surveillance data confirm multiyear predictions of rotavirus dynamics in New York City
- PMID: 32133392
- PMCID: PMC7043922
- DOI: 10.1126/sciadv.aax0586
Surveillance data confirm multiyear predictions of rotavirus dynamics in New York City
Abstract
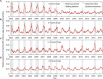
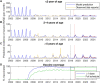
Prediction skill is a key test of models for epidemic dynamics. However, future validation of models against out-of-sample data is rare, partly because of a lack of timely surveillance data. We address this gap by analyzing the response of rotavirus dynamics to infant vaccination. Syndromic surveillance of emergency department visits for diarrhea in New York City reveals a marked decline in diarrheal incidence among infants and young children, in line with data on rotavirus-coded hospitalizations and laboratory-confirmed cases, and a shift from annual to biennial epidemics increasingly affecting older children and adults. A published mechanistic model qualitatively predicted these patterns more than 2 years in advance. Future efforts to increase vaccination coverage may disrupt these patterns and lead to further declines in the incidence of rotavirus-attributable gastroenteritis.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures

References
-
- Parashar U. D., Glass R. I., Rotavirus vaccines—Early success, remaining questions. N. Engl. J. Med. 360, 1063–1065 (2009). - PubMed
-
- Turcios R. M., Curns A. T., Holman R. C., Pandya-Smith I., LaMonte A., Bresee J. S., Glass R. I.; National Respiratory and Enteric Virus Surveillance System Collaborating Laboratories , Temporal and geographic trends of rotavirus activity in the United States, 1997–2004. Pediatr. Infect. Dis. J. 25, 451–454 (2006). - PubMed
-
- Curns A. T., Panozzo C. A., Tate J. E., Payne D. C., Patel M. M., Cortese M. M., Parashar U. D., Remarkable postvaccination spatiotemporal changes in United States rotavirus activity. Pediatr. Infect. Dis. J. 30, S54–S55 (2011). - PubMed
-
- Cortese M. M., Tate J. E., Simonsen L., Edelman L., Parashar U. D., Reduction in gastroenteritis in United States children and correlation with early rotavirus vaccine uptake from national medical claims databases. Pediatr. Infect. Dis. J. 29, 489–494 (2010). - PubMed
-
- Lopman B. A., Curns A. T., Yen C., Parashar U. D., Infant rotavirus vaccination may provide indirect protection to older children and adults in the United States. J. Infect. Dis. 204, 980–986 (2011). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

