Annexin A1 accounts for an anti-inflammatory binding target of sesamin metabolites
- PMID: 32133417
- PMCID: PMC7033200
- DOI: 10.1038/s41538-020-0064-6
Annexin A1 accounts for an anti-inflammatory binding target of sesamin metabolites
Abstract
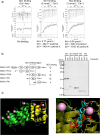
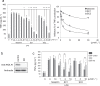
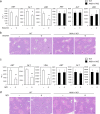
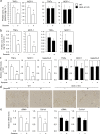
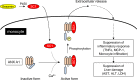
Sesamin [(7α,7'α,8α,8'α)-3,4:3',4'-bis(methylenedioxy)-7,9':7',9-diepoxylignane] is a major lignan in sesame seeds. Sesamin is converted to the catechol metabolite, SC1 [(7α,7'α,8α,8'α)-3',4'-methylenedioxy-7,9':7',9-diepoxylignane-3,4-diol] with anti-inflammatory effects after oral administration. However, its molecular target remains unknown. Analysis using high-performance affinity nanobeads led to the identification of annexin A1 (ANX A1) as an SC1-binding protein. SC1 was found to bind to the annexin repeat 3 region of ANX A1 with a high-affinity constant (Kd = 2.77 μmol L-1). In U937 cells, SC1 exhibited an anti-inflammatory effect dependent on ANX A1. Furthermore, administration of sesamin or SC1 attenuated carbon tetrachloride-induced liver damage in mice and concurrently suppressed inflammatory responses dependent on ANX A1. The mechanism involved SC1-induced ANX A1 phosphorylation at serine 27 that facilitates extracellular ANX A1 release. Consequently, the ANX A1 released into the extracellular space suppressed the production of tumor necrosis factor α. This study demonstrates that ANX A1 acts as a pivotal target of sesamin metabolites to attenuate inflammatory responses.
Keywords: Mechanism of action; Proteins.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsD.T., Y.O., S.A., Y.K., T.R. and H.S. are employees of Suntory Wellness Ltd, which is a manufacturer of foods that contain sesamin. All authors declare no other competing interests.
Figures


Similar articles
-
Sesamin Catechol Glucuronides Exert Anti-inflammatory Effects by Suppressing Interferon β and Inducible Nitric Oxide Synthase Expression through Deconjugation in Macrophage-like J774.1 Cells.J Agric Food Chem. 2019 Jul 10;67(27):7640-7649. doi: 10.1021/acs.jafc.8b07227. Epub 2019 Apr 16. J Agric Food Chem. 2019. PMID: 30951310
-
Identification of the metabolites of episesamin in rat bile and human liver microsomes.Biol Pharm Bull. 2012;35(5):709-16. doi: 10.1248/bpb.35.709. Biol Pharm Bull. 2012. PMID: 22687406
-
Lignan derivatives from the stem bark of Syzygium cumini (L.) Skeels.Nat Prod Res. 2009;23(5):422-30. doi: 10.1080/14786410802038697. Nat Prod Res. 2009. PMID: 19296384
-
Cracking the code of Annexin A1-mediated chemoresistance.Biochem Biophys Res Commun. 2024 Sep 17;725:150202. doi: 10.1016/j.bbrc.2024.150202. Epub 2024 May 31. Biochem Biophys Res Commun. 2024. PMID: 38885563 Review.
-
Macrophage biology in the Anx-A1-/- mouse.Prostaglandins Leukot Essent Fatty Acids. 2005 Feb;72(2):95-103. doi: 10.1016/j.plefa.2004.10.008. Prostaglandins Leukot Essent Fatty Acids. 2005. PMID: 15626592 Review.
Cited by
-
Inhibition of mitochondrial LonP1 protease by allosteric blockade of ATP binding and hydrolysis via CDDO and its derivatives.J Biol Chem. 2022 Mar;298(3):101719. doi: 10.1016/j.jbc.2022.101719. Epub 2022 Feb 11. J Biol Chem. 2022. PMID: 35151690 Free PMC article.
-
Glycyrrhizin Derivatives Suppress Cancer Chemoresistance by Inhibiting Progesterone Receptor Membrane Component 1.Cancers (Basel). 2021 Jun 29;13(13):3265. doi: 10.3390/cancers13133265. Cancers (Basel). 2021. PMID: 34209885 Free PMC article.
-
Transgenic Forsythia plants expressing sesame cytochrome P450 produce beneficial lignans.Sci Rep. 2022 Jun 16;12(1):10152. doi: 10.1038/s41598-022-14401-9. Sci Rep. 2022. PMID: 35710718 Free PMC article.
-
Production of beneficial lignans in heterologous host plants.Front Plant Sci. 2022 Oct 11;13:1026664. doi: 10.3389/fpls.2022.1026664. eCollection 2022. Front Plant Sci. 2022. PMID: 36330251 Free PMC article. No abstract available.
-
Sesamin Induces the Transdifferentiation of Type II Alveolar Epithelial Cells via AnnexinA1 and TRPV1.Lung. 2023 Feb;201(1):65-77. doi: 10.1007/s00408-023-00598-7. Epub 2023 Feb 3. Lung. 2023. PMID: 36735045
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

