The effect of maslinic acid on cognitive dysfunction induced by cholinergic blockade in mice
- PMID: 32133639
- PMCID: PMC7312314
- DOI: 10.1111/bph.15042
The effect of maslinic acid on cognitive dysfunction induced by cholinergic blockade in mice
Abstract
Background and purpose: Alzheimer's disease (AD) is the most prevalent disease associated with cognitive dysfunction. Current AD therapeutic agents have several gastrointestinal or psychological adverse effects and therefore, novel therapeutic agents with fewer adverse effects must be developed. Previously, we demonstrated that oleanolic acid, which is similar in chemical structure to maslinic acid, ameliorates cognitive impairment through the activation of tropomyosin receptor kinase (TrkB)-ERK-cAMP response element-binding protein (CREB) phosphorylation and increased levels of brain-derived neurotrophic factor (BDNF). In the present study, we investigate the effect of maslinic acid on cholinergic blockade-induced memory impairment in mice.
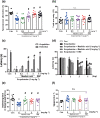
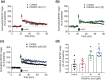
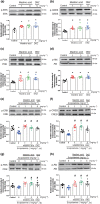
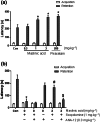
Methods and key results: Maslinic acid reversed scopolamine-induced memory impairment, as determined by the Y-maze, passive avoidance and Morris water maze tests. In addition, we also observed that ERK-CREB, PI3K and PKB (Akt) phosphorylation levels were increased by maslinic acid administration in the mouse hippocampus. Moreover, we determined that the effects of maslinic acid on scopolamine-induced memory impairment in the passive avoidance test were abolished by a specific TrkB receptor antagonist (ANA-12). Additionally, we observed similar temporal changes in the expression levels between BDNF and tissue plasminogen activator in the hippocampus.
Conclusion and implications: These findings suggest that maslinic acid enhances cognitive function through the activation of BDNF and its downstream pathway signalling in the hippocampus and that it might be a potential therapeutic agent for cognitive decline, such as that observed in AD.
© 2020 The British Pharmacological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Anisman, H. (1975). Dissociation of disinhibitory effects of scopolamine: Strain and task factors. Pharmacology, Biochemistry, and Behavior, 3, 613–618. - PubMed
-
- Ballatore, C. , Lee, V. M. , & Trojanowski, J. Q. (2007). Tau‐mediated neurodegeneration in Alzheimer's disease and related disorders. Nature Reviews. Neuroscience, 8, 663–672. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

