Loss-of-function of Endothelin receptor type A results in Oro-Oto-Cardiac syndrome
- PMID: 32133772
- PMCID: PMC7202054
- DOI: 10.1002/ajmg.a.61531
Loss-of-function of Endothelin receptor type A results in Oro-Oto-Cardiac syndrome
Abstract
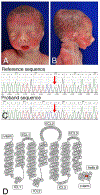
Craniofacial morphogenesis is regulated in part by signaling from the Endothelin receptor type A (EDNRA). Pathogenic variants in EDNRA signaling pathway components EDNRA, GNAI3, PCLB4, and EDN1 cause Mandibulofacial Dysostosis with Alopecia (MFDA), Auriculocondylar syndrome (ARCND) 1, 2, and 3, respectively. However, cardiovascular development is normal in MFDA and ARCND individuals, unlike Ednra knockout mice. One explanation may be that partial EDNRA signaling remains in MFDA and ARCND, as mice with reduced, but not absent, EDNRA signaling also lack a cardiovascular phenotype. Here we report an individual with craniofacial and cardiovascular malformations mimicking the Ednra -/- mouse phenotype, including a distinctive micrognathia with microstomia and a hypoplastic aortic arch. Exome sequencing found a novel homozygous missense variant in EDNRA (c.1142A>C; p.Q381P). Bioluminescence resonance energy transfer assays revealed that this amino acid substitution in helix 8 of EDNRA prevents recruitment of G proteins to the receptor, abrogating subsequent receptor activation by its ligand, Endothelin-1. This homozygous variant is thus the first reported loss-of-function EDNRA allele, resulting in a syndrome we have named Oro-Oto-Cardiac Syndrome. Further, our results illustrate that EDNRA signaling is required for both normal human craniofacial and cardiovascular development, and that limited EDNRA signaling is likely retained in ARCND and MFDA individuals. This work illustrates a straightforward approach to identifying the functional consequence of novel genetic variants in signaling molecules associated with malformation syndromes.
Keywords: Auriculocondylar syndrome; BRET; cardiovascular; micrognathia; neural crest cell.
© 2020 Wiley Periodicals, Inc.
Figures
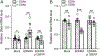



References
-
- Clouthier DE, Hosoda K, Richardson JA, Williams SC, Yanagisawa H, Kuwaki T, … Yanagisawa M. (1998). Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development, 125, 813–824.
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

