Hematoma enlargement characteristics in deep versus lobar intracerebral hemorrhage
- PMID: 32133793
- PMCID: PMC7086015
- DOI: 10.1002/acn3.51001
Hematoma enlargement characteristics in deep versus lobar intracerebral hemorrhage
Abstract
Objective: Hematoma enlargement (HE) is associated with clinical outcomes after supratentorial intracerebral hemorrhage (ICH). This study evaluates whether HE characteristics and association with functional outcome differ in deep versus lobar ICH.
Methods: Pooled analysis of individual patient data between January 2006 and December 2015 from a German-wide cohort study (RETRACE, I + II) investigating ICH related to oral anticoagulants (OAC) at 22 participating centers, and from one single-center registry (UKER-ICH) investigating non-OAC-ICH patients. Altogether, 1954 supratentorial ICH patients were eligible for outcome analyses, which were separately conducted or controlled for OAC, that is, vitamin-K-antagonists (VKA, n = 1186) and non-vitamin-K-antagonist-oral-anticoagulants (NOAC, n = 107). Confounding was addressed using propensity score matching, cox regression modeling and multivariate modeling. Main outcomes were occurrence, extent, and timing of HE (>33%/>6 mL) and its association with 3-month functional outcome.
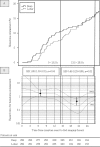
Results: Occurrence of HE was not different after deep versus lobar ICH in patients with non-OAC-ICH (39/356 [11.0%] vs. 36/305 [11.8%], P = 0.73), VKA-ICH (249/681 [36.6%] vs. 183/505 [36.2%], P = 0.91), and NOAC-ICH (21/69 [30.4%] vs. 12/38 [31.6%], P = 0.90). HE extent did not differ after non-OAC-ICH (deep:+59% [40-122] vs. lobar:+74% [37-124], P = 0.65), but both patients with VKA-ICH and NOAC-ICH showed greater HE extent after deep ICH [VKA-ICH, deep: +94% [54-199] vs. lobar: +56% [35-116], P < 0.001; NOAC-ICH, deep: +74% [56-123] vs. lobar: +40% [21-49], P = 0.001). Deep compared to lobar ICH patients had higher HE hazard during first 13.5 h after onset (Hazard ratio [HR]: 1.85 [1.03-3.31], P = 0.04), followed by lower hazard (13.5-26.5 h, HR: 0.46 [0.23-0.89], P = 0.02), and equal hazard thereafter (HR: 0.96 [0.56-1.65], P = 0.89). Odds ratio for unfavorable outcome was higher after HE in deep (4.31 [2.71-6.86], P < 0.001) versus lobar ICH (2.82 [1.71-4.66], P < 0.001), and only significant after small-medium (1st volume-quarter, deep: 3.09 [1.52-6.29], P < 0.01; lobar: 3.86 [1.35-11.04], P = 0.01) as opposed to large-sized ICH (4th volume-quarter, deep: 1.09 [0.13-9.20], P = 0.94; lobar: 2.24 [0.72-7.04], P = 0.17).
Interpretation: HE occurrence does not differ among deep and lobar ICH. However, compared to lobar ICH, HE after deep ICH is of greater extent in OAC-ICH, occurs earlier and may be of greater clinical relevance. Overall, clinical significance is more apparent after small-medium compared to large-sized bleedings.
© 2020 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals, Inc on behalf of American Neurological Association.
Conflict of interest statement
The authors have no conflicts of interest related to the contents of the manuscript.
Figures



References
-
- Flibotte JJ, Hagan N, O'Donnell J, et al. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology 2004;28:1059–1064. - PubMed
-
- Kuramatsu JB, Gerner ST, Schellinger PD, et al. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation‐related intracerebral hemorrhage. JAMA 2015;24:824–836. - PubMed
-
- Dowlatshahi D, Yogendrakumar V, Aviv RI, et al. Small intracerebral hemorrhages have a low spot sign prevalence and are less likely to expand. Int J Stroke 2016;11:191–197. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
