Inhibition of chemotherapy-related breast tumor EMT by application of redox-sensitive siRNA delivery system CSO-ss-SA/siRNA along with doxorubicin treatment
- PMID: 32133799
- PMCID: PMC7086009
- DOI: 10.1631/jzus.B1900468
Inhibition of chemotherapy-related breast tumor EMT by application of redox-sensitive siRNA delivery system CSO-ss-SA/siRNA along with doxorubicin treatment
Erratum in
-
Erratum to: Inhibition of chemotherapy-related breast tumor EMT by application of redox-sensitive siRNA delivery system CSO-ss-SA/siRNA along with doxorubicin treatment.J Zhejiang Univ Sci B. 2022 Feb 15;23(2):171-172. doi: 10.1631/jzus.B19e0468. J Zhejiang Univ Sci B. 2022. PMID: 35187891 Free PMC article. English.
Abstract
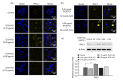
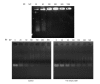
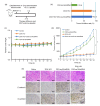
Metastasis is one of the main reasons causing death in cancer patients. It was reported that chemotherapy might induce metastasis. In order to uncover the mechanism of chemotherapy-induced metastasis and find solutions to inhibit treatment-induced metastasis, the relationship between epithelial-mesenchymal transition (EMT) and doxorubicin (DOX) treatment was investigated and a redox-sensitive small interfering RNA (siRNA) delivery system was designed. DOX-related reactive oxygen species (ROS) were found to be responsible for the invasiveness of tumor cells in vitro, causing enhanced EMT and cytoskeleton reconstruction regulated by Ras-related C3 botulinum toxin substrate 1 (RAC1). In order to decrease RAC1, a redox-sensitive glycolipid drug delivery system (chitosan-ss-stearylamine conjugate (CSO-ss-SA)) was designed to carry siRNA, forming a gene delivery system (CSO-ss-SA/siRNA) downregulating RAC1. CSO-ss-SA/siRNA exhibited an enhanced redox sensitivity compared to nonresponsive complexes in 10 mmol/L glutathione (GSH) and showed a significant safety. CSO-ss-SA/siRNA could effectively transmit siRNA into tumor cells, reducing the expression of RAC1 protein by 38.2% and decreasing the number of tumor-induced invasion cells by 42.5%. When combined with DOX, CSO-ss-SA/siRNA remarkably inhibited the chemotherapy-induced EMT in vivo and enhanced therapeutic efficiency. The present study indicates that RAC1 protein is a key regulator of chemotherapy-induced EMT and CSO-ss-SA/siRNA silencing RAC1 could efficiently decrease the tumor metastasis risk after chemotherapy.
Keywords: Doxorubicin; Tumor metastasis; Ras-related C3 botulinum toxin substrate 1 (RAC1); Epithelial-mesenchymal transition (EMT); Chitosan micelles; Small interfering RNA (siRNA).
Conflict of interest statement
All institutional and national guidelines for the care and use of laboratory animals were followed.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

