Down-regulation of protease-activated receptor 2 ameliorated osteoarthritis in rats through regulation of MAPK/NF-κB signaling pathway in vivo and in vitro
- PMID: 32134473
- PMCID: PMC7098131
- DOI: 10.1042/BSR20192620
Down-regulation of protease-activated receptor 2 ameliorated osteoarthritis in rats through regulation of MAPK/NF-κB signaling pathway in vivo and in vitro
Abstract
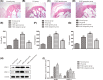
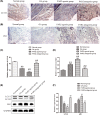
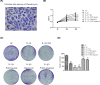
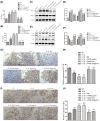
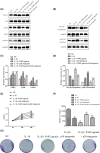
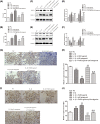
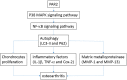
Recently, protease-activated receptor 2 (PAR2) has been proved to be involved in the inflammatory response including osteoarthritis (OA). In the present study, we found that PAR2 antagonist could remarkably improve the pathological condition of OA rats in vivo. In addition, we also found that PAR2 antagonist could suppress the production of inflammatory factors (TNF-α and Cox-2), decrease the levels of MMP-1 and MMP-13, and restrain the levels of P62 proteins and aggravate the expression of LC3-II both in vivo and in vitro. Besides, in vitro, PAR2 antagonist could increase the proliferation and colony formation of chondrocytes induced with IL-1β. Moreover, PAR2 antagonist could decrease the expression of expressions of p-p38, p-IκBα and p-NF-κB in vitro. However, PAR2 agonist exhibited the opposite effects. Furthermore, SB203580, a p38 MAPK inhibitor, could remarkably promote the proliferation of chondrocytes induced with IL-1β, could alleviate the production of TNF-α and Cox-2, could down-regulate the protein expressions of MMP-1 and MMP-13, and could decrease the expression of P62 and increase the expressions of LC3-II of chondrocytes induced with IL-1β. Importantly, SB203580 could reverse the effects of PAR2 agonist on the functions of chondrocytes induced with IL-1β. Taken together, the present data suggest that down-regulation of PAR2 can ameliorate OA through inducing autophagy via regulation of MAPK/NF-κB signaling pathway in vivo and in vitro, and PAR2 can be considered as a potential candidate to treat OA.
Keywords: Autophagy; MAPK/NF-kB signaling pathway; Osteoarthritis; Protease-activated receptor 2.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

