The Status of Vaccine Development Against the Human Cytomegalovirus
- PMID: 32134478
- PMCID: PMC7057802
- DOI: 10.1093/infdis/jiz447
The Status of Vaccine Development Against the Human Cytomegalovirus
Abstract
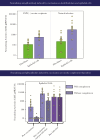
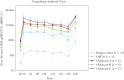
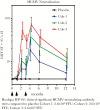
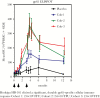
Numerous candidate vaccines against cytomegalovirus (CMV) infection and disease are in development. Whereas the previous article [1] provides background and opinions about the issues relating to vaccination, this article provides specifics about the vaccines in active development, as reported at a National Institutes of Health-sponsored meeting in Bethesda on September 4-6, 2018. Here, vaccine developers provide synopses of their candidate vaccines to immunize women to protect against congenital CMV disease and to prevent the consequences of CMV disease in recipients of transplanted organs or hematopoietic stem calls. The projects are presented here roughly in the descending order of their stage of development in the opinion of the first author.
Keywords: cytomegalovirus; fetal infection; organ transplant; stem cell transplant.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures

References
-
- Plotkin SA. Can Infection by the human Cytomegalovirus be Prevented? J Infect Dis 2019.
-
- Wang D, Freed DC, He X, et al. A replication-defective human cytomegalovirus vaccine for prevention of congenital infection. Sci Transl Med 2016; 8:362ra145. - PubMed
-
- Adler SP, Lewis N, Conlon A, et al. Phase 1 clinical trial of a conditionally replication-defective human cytomegalovirus (CMV) vaccine in CMV-seronegative subjects. J Infect Dis 2019; 220:411–9. - PubMed
-
- Gonczol E, Ianacone J, Ho WZ, Starr S, Meignier B, Plotkin S. Isolated gA/gB glycoprotein complex of human cytomegalovirus envelope induces humoral and cellular immune-responses in human volunteers. Vaccine 1990; 8:130–6. - PubMed

