On-Surface Synthesis of NBN-Doped Zigzag-Edged Graphene Nanoribbons
- PMID: 32134547
- PMCID: PMC7318338
- DOI: 10.1002/anie.202000488
On-Surface Synthesis of NBN-Doped Zigzag-Edged Graphene Nanoribbons
Abstract
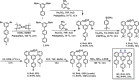
We report the first bottom-up synthesis of NBN-doped zigzag-edged GNRs (NBN-ZGNR1 and NBN-ZGNR2) through surface-assisted polymerization and cyclodehydrogenation based on two U-shaped molecular precursors with an NBN unit preinstalled at the zigzag edge. The resultant zigzag-edge topologies of GNRs are elucidated by high-resolution scanning tunneling microscopy (STM) in combination with noncontact atomic force microscopy (nc-AFM). Scanning tunneling spectroscopy (STS) measurements and density functional theory (DFT) calculations reveal that the electronic structures of NBN-ZGNR1 and NBN-ZGNR2 are significantly different from those of their corresponding pristine fully-carbon-based ZGNRs. Additionally, DFT calculations predict that the electronic structures of NBN-ZGNRs can be further tailored to be gapless and metallic through one-electron oxidation of each NBN unit into the corresponding radical cations. This work reported herein provides a feasible strategy for the synthesis of GNRs with stable zigzag edges yet tunable electronic properties.
Keywords: NBN doping; graphene nanoribbons; on-surface synthesis; radical cations; zigzag edges.
© 2020 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- None
-
- Cai J., Ruffieux P., Jaafar R., Bieri M., Braun T., Blankenburg S., Muoth M., Seitsonen A. P., Saleh M., Feng X., Müllen K., Fasel R., Nature 2010, 466, 470; - PubMed
-
- Chen L., Hernandez Y., Feng X., Müllen K., Angew. Chem. Int. Ed. 2012, 51, 7640–7654; - PubMed
- Angew. Chem. 2012, 124, 7758–7773;
-
- Slota M., Keerthi A., Myers W. K., Tretyakov E., Baumgarten M., Ardavan A., Sadeghi H., Lambert C. J., Narita A., Müllen K., Bogani L., Nature 2018, 557, 691–695; - PubMed
-
- Son Y.-W., Cohen M. L., Louie S. G., Nature 2006, 444, 347–349; - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

