Hypoxia potentiates the capacity of melanoma cells to evade cisplatin and doxorubicin cytotoxicity via glycolytic shift
- PMID: 32134564
- PMCID: PMC7193165
- DOI: 10.1002/2211-5463.12830
Hypoxia potentiates the capacity of melanoma cells to evade cisplatin and doxorubicin cytotoxicity via glycolytic shift
Abstract
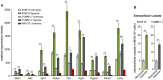
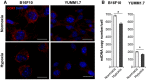
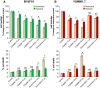
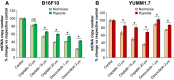
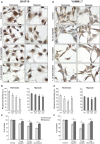
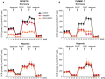
The hypoxic environment within solid tumors impedes the efficacy of chemotherapeutic treatments. Here, we demonstrate that hypoxia augments the capacity of melanoma cells to withstand cisplatin and doxorubicin cytotoxicity. We show that B16F10 cells derived from spontaneously formed melanoma and YUMM1.7 cells, engineered to recapitulate human-relevant melanoma driver mutations, profoundly differ in their vulnerabilities to cisplatin and doxorubicin. The differences are manifested in magnitude of proliferative arrest and cell death rates, extent of mtDNA depletion, and impairment of mitochondrial respiration. In both models, cytotoxicity is mitigated by hypoxia, which augments glycolytic metabolism. Collectively, the findings implicate metabolic reprogramming in drug evasion and suggest that melanoma tumors with distinct genetic makeup may have differential drug vulnerabilities, highlighting the importance of precision anticancer treatments.
Keywords: cisplatin; doxorubicin; glycolysis; hypoxia; melanoma; mitochondrial respiration.
© 2020 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Medical
Research Materials

