Selective Oxidation by H5[PV2Mo10O40] in a Highly Acidic Medium
- PMID: 32134633
- PMCID: PMC7482320
- DOI: 10.1021/acs.inorgchem.9b03747
Selective Oxidation by H5[PV2Mo10O40] in a Highly Acidic Medium
Abstract
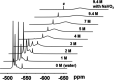
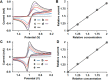
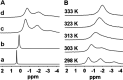
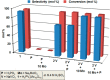
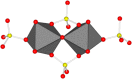
Dissolution of the polyoxometalate (POM) cluster anion H5[PV2Mo10O40] (1; a mixture of positional isomers) in 50% aq H2SO4 dramatically enhances its ability to oxidize methylarenes, while fully retaining the high selectivities typical of this versatile oxidant. To better understand this impressive reactivity, we now provide new information regarding the nature of 1 (115 mM) in 50% (9.4 M) H2SO4. Data from 51V NMR spectroscopy and cyclic voltammetry reveal that as the volume of H2SO4 in water is incrementally increased to 50%, V(V) ions are stoichiometrically released from 1, generating two reactive pervanadyl, VO2+, ions, each with a one-electron reduction potential of ca. 0.95 V (versus Ag/AgCl), compared to 0.46 V for 1 in 1.0 M aq H2SO4. Phosphorus-31 NMR spectra obtained in parallel reveal the presence of PO43-, which at 50% H2SO4 accounts for all the P(V) initially present in 1. Addition of (NH4)2SO4 leads to the formation of crystalline [NH4]6[Mo2O5(SO4)4] (34% yield based on Mo), whose structure (from single-crystal X-ray diffraction) features a corner-shared, permolybdenyl [Mo2O5]2+ core, conceptually derived by acid condensation of two MoO3 moieties. While 1 in 50% aq H2SO4 oxidizes p-xylene to p-methylbenzaldehyde with conversion and selectivity both greater than 90%, reaction with VO2+ alone gives the same high conversion, but at a significantly lower selectivity. Importantly, selectivity is fully restored by adding [NH4]6[Mo2O5(SO4)4], suggesting a central role for Mo(VI) in attenuating the (generally) poor selectivity achievable using VO2+ alone. Finally, 31P and 51V NMR spectra show that intact 1 is fully restored upon dilution to 1 M H2SO4.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Spectroscopic Study of the Behavior of Mo(VI) and W(VI) Polyanions in Sulfuric-Phosphoric Acid Mixtures.Inorg Chem. 2021 Dec 6;60(23):17565-17578. doi: 10.1021/acs.inorgchem.1c02133. Epub 2021 Nov 5. Inorg Chem. 2021. PMID: 34738803
-
Syntheses and characterization of phosphonates and diphosphonates of molybdenum, A4[(MoO3)5(O3PR)2]·xH2O, A2[Mo2O5(O3PR)2] and A2[Mo2O5(O3P-R-PO3)] (A = K, Rb, Cs, Tl, NH4).Dalton Trans. 2017 Nov 28;46(46):16102-16112. doi: 10.1039/c7dt03012f. Dalton Trans. 2017. PMID: 29125165
-
Hexa- and dodecanuclear polyoxomolybdate cyclic compounds: application toward the facile synthesis of nanoparticles and film electrodeposition.Chemistry. 2009;15(3):733-41. doi: 10.1002/chem.200800719. Chemistry. 2009. PMID: 19040248
-
Synthesis and characterization of novel Wells-Dawson-type mono vanadium(V)-substituted tungsto-polyoxometalate isomers: 1- and 4-[S2VW17O62](5-).Inorg Chem. 2014 May 19;53(10):4891-8. doi: 10.1021/ic402994q. Epub 2014 Apr 30. Inorg Chem. 2014. PMID: 24784547
-
An evaluation of structural models for the photosynthetic water-oxidizing complex derived from spectroscopic and X-ray diffraction signatures.J Biol Inorg Chem. 2002 Jan;7(1-2):2-22. doi: 10.1007/s00775-001-0305-3. Epub 2001 Nov 8. J Biol Inorg Chem. 2002. PMID: 11862536 Review.
References
-
- Khenkin A. M.; Neumann R. Low-temperature activation of dioxygen and hydrocarbon oxidation catalyzed by a phosphovanadomolybdate: evidence for a Mars-van Krevelen type mechanism in a homogeneous liquid phase. Angew. Chem., Int. Ed. 2000, 39, 4088–4090. 10.1002/1521-3773(20001117)39:22<4088::AID-ANIE4088>3.0.CO;2-#. - DOI - PubMed
-
- Khenkin A. M.; Weiner L.; Wang Y.; Neumann R. Electron and oxygen transfer in polyoxometalate, H5PV2Mo10O40, catalyzed oxidation of aromatic and alkyl aromatic compounds: Evidence for aerobic Mars-van Krevelen-Type reactions in the liquid homogeneous phase. J. Am. Chem. Soc. 2001, 123, 8531–8542. 10.1021/ja004163z. - DOI - PubMed
-
- Reichert J.; Brunner B.; Jess A.; Wasserscheid P.; Albert J. Biomass oxidation to formic acid in aqueous media using polyoxometalate catalysts - boosting FA selectivity by in-situ extraction. Energy Environ. Sci. 2015, 8, 2985–2990. 10.1039/C5EE01706H. - DOI
LinkOut - more resources
Full Text Sources

