Mapping of Ion and Substrate Binding Sites in Human Sodium Iodide Symporter (hNIS)
- PMID: 32134653
- PMCID: PMC7249729
- DOI: 10.1021/acs.jcim.9b01114
Mapping of Ion and Substrate Binding Sites in Human Sodium Iodide Symporter (hNIS)
Abstract
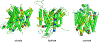
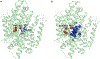
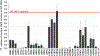
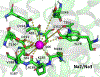
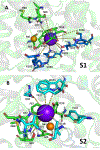
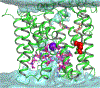
The human sodium iodide symporter (hNIS) is a theranostic reporter gene which concentrates several clinically approved SPECT and PET radiotracers and plays an essential role for the synthesis of thyroid hormones as an iodide transporter in the thyroid gland. Development of hNIS mutants which could enhance translocation of the desired imaging ions is currently underway. Unfortunately, it is hindered by lack of understanding of the 3D organization of hNIS and its relation to anion transport. There are no known crystal structures of hNIS in any of its conformational states. Homology modeling can be very effective in such situations; however, the low sequence identity between hNIS and relevant secondary transporters with available experimental structures makes the choice of a template and the generation of 3D models nontrivial. Here, we report a combined application of homology modeling and molecular dynamics refining of the hNIS structure in its semioccluded state. The modeling was based on templates from the LeuT-fold protein family and was done with emphasis on the refinement of the substrate-ion binding pocket. The consensus model developed in this work is compared to available biophysical and biochemical experimental data for a number of different LeuT-fold proteins. Some functionally important residues contributing to the formation of putative binding sites and permeation pathways for the cotransported Na+ ions and I- substrate were identified. The model predictions were experimentally tested by generation of mutant versions of hNIS and measurement of relative (to WT hNIS) 125I- uptake of 35 hNIS variants.
Conflict of interest statement
The authors declare the following competing financial interest(s): T.S., R.J, P.J.L, and L.S. are Imanis employees. S.J.R, K.-W.P, and the Mayo Clinic have a financial interest in the NIS technology and Imanis Life Sciences. The other authors declare that they have no competing interests.
Figures
References
-
- Wright EM; Turk E The Sodium/Glucose Cotransport Family SLC5. Pfluegers Arch. 2004, 447 (5), 510–8. - PubMed
-
- Darrouzet E; Lindenthal S; Marcellin D; Pellequer JL; Pourcher T The Sodium/Iodide Symporter: State of the Art of Its Molecular Characterization. Biochim. Biophys. Acta, Biomembr 2014, 1838 (1), 244–253. - PubMed
-
- Eskandari S; Loo DD; Dai G; Levy O; Wright EM; Carrasco N Thyroid Na+/I- Symporter. Mechanism, Stoichiometry, and Specificity. J. Biol. Chem 1997, 272 (43), 27230–8. - PubMed
-
- Van Sande J; Massart C; Beauwens R; Schoutens A; Costagliola S; Dumont JE; Wolff J Anion Selectivity by the Sodium Sodide Symporter. Endocrinology 2003, 144 (1), 247–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

