Activity of Vincristine and Irinotecan in Diffuse Anaplastic Wilms Tumor and Therapy Outcomes of Stage II to IV Disease: Results of the Children's Oncology Group AREN0321 Study
- PMID: 32134700
- PMCID: PMC7213587
- DOI: 10.1200/JCO.19.01265
Activity of Vincristine and Irinotecan in Diffuse Anaplastic Wilms Tumor and Therapy Outcomes of Stage II to IV Disease: Results of the Children's Oncology Group AREN0321 Study
Abstract
Purpose: AREN0321 evaluated the activity of vincristine and irinotecan (VI) in patients with newly diagnosed diffuse anaplastic Wilms tumor (DAWT) and whether a regimen containing carboplatin (regimen UH1) in addition to regimen I agents used in the National Wilms Tumor Study 5 (NWTS-5; vincristine, doxorubicin, cyclophosphamide, and etoposide plus radiotherapy) would improve patient outcomes.
Patients and methods: Patients with stage II to IV DAWT without measurable disease received regimen UH1. Patients with stage IV measurable disease were eligible to receive VI (vincristine, 1.5 mg/m2 per day intravenously on days 1 and 8; irinotecan, 20 mg/m2 per day intravenously on days 1-5 and 8-12 of a 21-day cycle) in an upfront window; those with complete (CR) or partial response (PR) had VI incorporated into regimen UH1 (regimen UH2). The study was designed to detect improvement in outcomes of patients with stage II to IV DAWT compared with historical controls treated with regimen I.
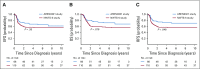
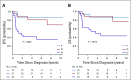
Results: Sixty-six eligible patients were enrolled. Of 14 patients with stage IV measurable disease who received VI, 11 (79%) achieved CR (n = 1) or PR (n = 10) after 2 cycles. Doses of doxorubicin, cyclophosphamide, and etoposide were reduced midstudy because of nonhematologic toxicity. Four patients (6%) died as a result of toxicity. Four-year event-free survival, relapse-free survival, and overall survival rates were 67.7% (95% CI, 55.9% to 79.4%), 72.9% (95% CI, 61.5% to 84.4%), and 73.7% (95% CI, 62.7% to 84.8%), respectively, compared with 57.5% (95% CI, 47.6% to 67.4%; P = .26), 57.5% (95% CI, 47.6% to 67.4%; P = .048), and 59.2% (95% CI, 49.4% to 69.0%; P = .08), respectively, in NWTS-5.
Conclusion: VI produced a high response rate in patients with metastatic DAWT. AREN0321 treatment seemed to improve outcomes for patients with stage II to IV DAWT compared with NWTS-5, but with increased toxicity. The UH2 regimen warrants further investigation with modifications to reduce toxicity.
Figures
References
-
- Green DM, Beckwith JB, Breslow NE, et al. Treatment of children with stages II to IV anaplastic Wilms’ tumor: A report from the National Wilms’ Tumor Study Group. J Clin Oncol. 1994;12:2126–2131. - PubMed
-
- Vujanić GM, Harms D, Sandstedt B, et al. New definitions of focal and diffuse anaplasia in Wilms tumor: The International Society of Paediatric Oncology (SIOP) experience. Med Pediatr Oncol. 1999;32:317–323. - PubMed
-
- Weirich A, Ludwig R, Graf N, et al. Survival in nephroblastoma treated according to the trial and study SIOP-9/GPOH with respect to relapse and morbidity. Ann Oncol. 2004;15:808–820. - PubMed
-
- Verschuur A, Van Tinteren H, Graf N, et al. Treatment of pulmonary metastases in children with stage IV nephroblastoma with risk-based use of pulmonary radiotherapy. J Clin Oncol. 2012;30:3533–3539. - PubMed
-
- Dome JS, Cotton CA, Perlman EJ, et al. Treatment of anaplastic histology Wilms’ tumor: Results from the fifth National Wilms’ Tumor Study. J Clin Oncol. 2006;24:2352–2358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

