Differentiation of human colon tissue in culture: Effects of calcium on trans-epithelial electrical resistance and tissue cohesive properties
- PMID: 32134920
- PMCID: PMC7058309
- DOI: 10.1371/journal.pone.0222058
Differentiation of human colon tissue in culture: Effects of calcium on trans-epithelial electrical resistance and tissue cohesive properties
Abstract
Background and aims: Human colonoid cultures maintained under low-calcium (0.25 mM) conditions undergo differentiation spontaneously and, concomitantly, express a high level of tight junction proteins, but not desmosomal proteins. When calcium is included to a final concentration of 1.5-3.0 mM (provided either as a single agent or as a combination of calcium and additional minerals), there is little change in tight junction protein expression but a strong up-regulation of desmosomal proteins and an increase in desmosome formation. The aim of this study was to assess the functional consequences of calcium-mediated differences in barrier protein expression.
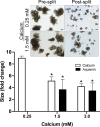
Methods: Human colonoid-derived epithelial cells were interrogated in transwell culture under low- or high-calcium conditions for monolayer integrity and ion permeability by measuring trans-epithelial electrical resistance (TEER) across the confluent monolayer. Colonoid cohesiveness was assessed in parallel.
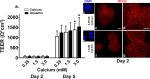
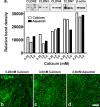
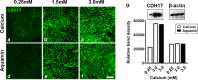
Results: TEER values were high in the low-calcium environment but increased in response to calcium. In addition, colonoid cohesiveness increased substantially with calcium supplementation. In both assays, the response to multi-mineral intervention was greater than the response to calcium alone. Consistent with these findings, several components of tight junctions were expressed at 0.25 mM calcium but these did not increase substantially with supplementation. Cadherin-17 and desmoglein-2, in contrast, were weakly-expressed under low calcium conditions but increased with intervention.
Conclusions: These findings indicate that low ambient calcium levels are sufficient to support the formation of a permeability barrier in the colonic epithelium. Higher calcium levels promote tissue cohesion and enhance barrier function. These findings may help explain how an adequate calcium intake contributes to colonic health by improving barrier function, even though there is little change in colonic histological features over a wide range of calcium intake levels.
Conflict of interest statement
There is no conflict of interest to declare from any author. Marigot LTD (Cork, Ireland) has provided Aquamin® as a gift for use in the study. This does not, in any way, alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

