Monophosphoryl lipid A alleviated radiation-induced testicular injury through TLR4-dependent exosomes
- PMID: 32135028
- PMCID: PMC7171420
- DOI: 10.1111/jcmm.14978
Monophosphoryl lipid A alleviated radiation-induced testicular injury through TLR4-dependent exosomes
Abstract
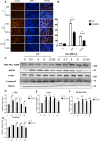
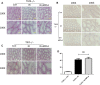
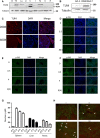
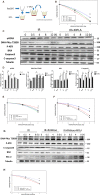
Radiation protection on male testis is an important task for ionizing radiation-related workers or people who receive radiotherapy for tumours near the testicle. In recent years, Toll-like receptors (TLRs), especially TLR4, have been widely studied as a radiation protection target. In this study, we detected that a low-toxicity TLR4 agonist monophosphoryl lipid A (MPLA) produced obvious radiation protection effects on mice testis. We found that MPLA effectively alleviated testis structure damage and cell apoptosis induced by ionizing radiation (IR). However, as the expression abundance differs a lot in distinct cells and tissues, MPLA seemed not to directly activate TLR4 singling pathway in mice testis. Here, we demonstrated a brand new mechanism for MPLA producing radiation protection effects on testis. We observed a significant activation of TLR4 pathway in macrophages after MPLA stimulation and identified significant changes in macrophage-derived exosomes protein expression. We proved that after MPLA treatment, macrophage-derived exosomes played an important role in testis radiation protection, and specially, G-CSF and MIP-2 in exosomes are the core molecules in this protection effect.
Keywords: MPLA; exosome; radioprotection; testis.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures



References
-
- Choy H, Gerber DE, Bradley JD, et al. Concurrent pemetrexed and radiation therapy in the treatment of patients with inoperable stage III non‐small cell lung cancer: a systematic review of completed and ongoing studies. Lung Cancer. 2015;87:232‐240. - PubMed
-
- Martin NE, D'Amico AV. Progress and controversies: Radiation therapy for prostate cancer. CA Cancer J Clin. 2014;64:389‐407. - PubMed
-
- Benson AB 3rd, Arnoletti JP, Bekaii‐Saab T, et al. Colon cancer. J Natl Compr Canc Netw. 2011;9:1238‐1290. - PubMed
-
- Bujan L, Walschaerts M, Moinard N, et al. Impact of chemotherapy and radiotherapy for testicular germ cell tumors on spermatogenesis and sperm DNA: a multicenter prospective study from the CECOS network. Fertil Steril. 2013;100:673‐680. - PubMed
-
- Grignard E, Gueguen Y, Grison S, et al. Testicular steroidogenesis is not altered by 137 cesium Chernobyl fallout, following in utero or post‐natal chronic exposure. C R Biol. 2010;333:416‐423. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

