Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor registry trial
- PMID: 32135080
- PMCID: PMC7453743
- DOI: 10.1016/S1470-2045(20)30074-7
Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor registry trial
Erratum in
-
Correction to Lancet Oncol 2020; 21: 508-18.Lancet Oncol. 2020 Apr;21(4):e182. doi: 10.1016/S1470-2045(20)30177-7. Lancet Oncol. 2020. PMID: 32240611 No abstract available.
Abstract
Background: About 25% of pancreatic cancers harbour actionable molecular alterations, defined as molecular alterations for which there is clinical or strong preclinical evidence of a predictive benefit from a specific therapy. The Know Your Tumor (KYT) programme includes US patients with pancreatic cancer and enables patients to undergo commercially available multi-omic profiling to provide molecularly tailored therapy options and clinical trial recommendations. We sought to determine whether patients with pancreatic cancer whose tumours harboured such actionable molecular alterations and who received molecularly matched therapy had a longer median overall survival than similar patients who did not receive molecularly matched therapy.
Methods: In this retrospective analysis, treatment history and longitudinal survival outcomes were analysed in patients aged 18 years or older with biopsy-confirmed pancreatic cancer of any stage, enrolled in the KYT programme and who received molecular testing results. Since the timing of KYT enrolment varied for each patient, the primary outcome measurement of median overall survival was calculated from the initial diagnosis of advanced disease until death. We compared median overall survival in patients with actionable mutations who were treated with a matched therapy versus those who were not treated with a matched therapy.
Findings: Of 1856 patients with pancreatic cancer who were referred to the KYT programme between June 16, 2014, and March 31, 2019, 1082 (58%) patients received personalised reports based on their molecular testing results. Actionable molecular alterations were identified in 282 (26%) of 1082 samples. Among 677 patients for whom outcomes were available, 189 had actionable molecular alterations. With a median follow-up of 383 days (IQR 214-588), those patients with actionable molecular alterations who received a matched therapy (n=46) had significantly longer median overall survival than did those patients who only received unmatched therapies (n=143; 2·58 years [95% CI 2·39 to not reached] vs 1·51 years [1·33-1·87]; hazard ratio 0·42 [95% CI 0·26-0·68], p=0·0004). The 46 patients who received a matched therapy also had significantly longer overall survival than the 488 patients who did not have an actionable molecular alteration (2·58 years [95% CI 2·39 to not reached] vs 1·32 years [1·25-1·47]; HR 0·34 [95% CI 0·22-0·53], p<0·0001). However, median overall survival did not differ between the patients who received unmatched therapy and those without an actionable molecular alteration (HR 0·82 [95% CI 0·64-1·04], p=0·10).
Interpretation: These real-world outcomes suggest that the adoption of precision medicine can have a substantial effect on survival in patients with pancreatic cancer, and that molecularly guided treatments targeting oncogenic drivers and the DNA damage response and repair pathway warrant further prospective evaluation.
Funding: Pancreatic Cancer Action Network and Perthera.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures
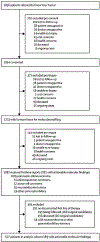
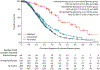
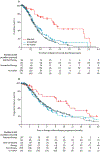


Comment in
-
Precision oncology for pancreatic cancer in real-world settings.Lancet Oncol. 2020 Apr;21(4):469-471. doi: 10.1016/S1470-2045(20)30148-0. Epub 2020 Mar 2. Lancet Oncol. 2020. PMID: 32135081 No abstract available.
-
Knowing your tumour?Lancet Oncol. 2020 Jun;21(6):e297. doi: 10.1016/S1470-2045(20)30283-7. Lancet Oncol. 2020. PMID: 32502449 No abstract available.
References
-
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014; 74: 2913–21. - PubMed
-
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817–25. - PubMed
-
- Bailey P, Chang DK, Nones K, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016; 531: 47–52. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

