Pathogenic DDX3X Mutations Impair RNA Metabolism and Neurogenesis during Fetal Cortical Development
- PMID: 32135084
- PMCID: PMC7331285
- DOI: 10.1016/j.neuron.2020.01.042
Pathogenic DDX3X Mutations Impair RNA Metabolism and Neurogenesis during Fetal Cortical Development
Abstract
De novo germline mutations in the RNA helicase DDX3X account for 1%-3% of unexplained intellectual disability (ID) cases in females and are associated with autism, brain malformations, and epilepsy. Yet, the developmental and molecular mechanisms by which DDX3X mutations impair brain function are unknown. Here, we use human and mouse genetics and cell biological and biochemical approaches to elucidate mechanisms by which pathogenic DDX3X variants disrupt brain development. We report the largest clinical cohort to date with DDX3X mutations (n = 107), demonstrating a striking correlation between recurrent dominant missense mutations, polymicrogyria, and the most severe clinical outcomes. We show that Ddx3x controls cortical development by regulating neuron generation. Severe DDX3X missense mutations profoundly disrupt RNA helicase activity, induce ectopic RNA-protein granules in neural progenitors and neurons, and impair translation. Together, these results uncover key mechanisms underlying DDX3X syndrome and highlight aberrant RNA metabolism in the pathogenesis of neurodevelopmental disease.
Keywords: DDX3X; autism; corpus callosum; cortical development; helicase; intellectual disability; polymicrogyria; radial glial progenitor; stress granule; translation.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures


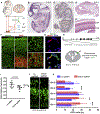
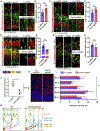

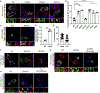

Comment in
-
Unwind and Relax: DDX3X RNA Helicase as a Critical Mediator of Cortical Neurogenesis.Neuron. 2020 May 6;106(3):357-358. doi: 10.1016/j.neuron.2020.03.035. Neuron. 2020. PMID: 32380046
References
-
- Achenbach TM (2011). Child Behavior Checklist. In Encyclopedia of Clinical Neuropsychology, Kreutzer JS, DeLuca J, and Caplan B, eds. (New York, NY: Springer New York; ), pp. 546–552.
-
- Amadei G, Zander MA, Yang G, Dumelie JG, Vessey JP, Lipshitz HD, Smibert CA, Kaplan DR, and Miller FD (2015). A Smaug2-Based Translational Repression Complex Determines the Balance between Precursor Maintenance versus Differentiation during Mammalian Neurogenesis. The Journal of neuroscience : the official journal of the Society for Neuroscience 35, 15666–15681. - PMC - PubMed
-
- Balak C, Benard M, Schaefer E, Iqbal S, Ramsey K, Ernoult-Lange M, Mattioli F, Llaci L, Geoffroy V, Courel M, et al. (2019). Rare De Novo Missense Variants in RNA Helicase DDX6 Cause Intellectual Disability and Dysmorphic Features and Lead to P-Body Defects and RNA Dysregulation. Am J Hum Genet 105, 509–525. - PMC - PubMed
-
- Beal B, Hayes I, McGaughran J, Amor DJ, Miteff C, Jackson V, van Reyk O, Subramanian G, Hildebrand MS, Morgan AT, et al. (2019). Expansion of phenotype of DDX3X syndrome: six new cases. Clinical dysmorphology 28, 169–174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

