Radiosensitization by Hyperthermia: The Effects of Temperature, Sequence, and Time Interval in Cervical Cell Lines
- PMID: 32138173
- PMCID: PMC7139900
- DOI: 10.3390/cancers12030582
Radiosensitization by Hyperthermia: The Effects of Temperature, Sequence, and Time Interval in Cervical Cell Lines
Abstract
Cervical cancers are almost exclusively caused by an infection with the human papillomavirus (HPV). When patients suffering from cervical cancer have contraindications for chemoradiotherapy, radiotherapy combined with hyperthermia is a good treatment option. Radiation-induced DNA breaks can be repaired by nonhomologous end-joining (NHEJ) or homologous recombination (HR). Hyperthermia can temporarily inactivate homologous recombination. Therefore, combining radiotherapy with hyperthermia can result in the persistence of more fatal radiation-induced DNA breaks. However, there is no consensus on the optimal sequence of radiotherapy and hyperthermia and the optimal time interval between these modalities. Moreover, the temperature of hyperthermia and HPV-type may also be important in radiosensitization by hyperthermia. In this study we thoroughly investigated the impact of different temperatures (37-42 °C), and the sequence of and time interval (0 up to 4 h) between ionizing radiation and hyperthermia on HPV16+: SiHa, Caski; HPV18+: HeLa, C4I; and HPV-: C33A, HT3 cervical cancer cell lines. Our results demonstrate that a short time interval between treatments caused more unrepaired DNA damages and more cell kill, especially at higher temperatures. Although hyperthermia before ionizing radiation may result in slightly more DNA damage, the sequence between hyperthermia and ionizing radiation yielded similar effects on cell survival.
Keywords: human papillomavirus; hyperthermia; ionizing radiation; sequence; time interval.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



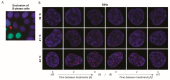



References
-
- Datta N.R., Ordonez S.G., Gaipl U.S., Paulides M.M., Crezee H., Gellermann J., Marder D., Puric E., Bodis S. Local hyperthermia combined with radiotherapy and-/or chemotherapy: Recent advances and promises for the future. Cancer Treat. Rev. 2015;41:742–753. doi: 10.1016/j.ctrv.2015.05.009. - DOI - PubMed
-
- Cihoric N., Tsikkinis A., van Rhoon G., Crezee H., Aebersold D.M., Bodis S., Beck M., Nadobny J., Budach V., Wust P., et al. Hyperthermia-related clinical trials on cancer treatment within the ClinicalTrials.gov registry. Int. J. Hyperthermia. 2015;31:609–614. doi: 10.3109/02656736.2015.1040471. - DOI - PubMed
-
- Datta N.R., Rogers S., Klingbiel D., Gomez S., Puric E., Bodis S. Hyperthermia and radiotherapy with or without chemotherapy in locally advanced cervical cancer: A systematic review with conventional and network meta-analyses. Int. J. Hyperthermia. 2016;32:809–821. doi: 10.1080/02656736.2016.1195924. - DOI - PubMed
-
- Lutgens L., van der Zee J., Pijls-Johannesma M., De Haas-Kock D.F., Buijsen J., Mastrigt G.A., Lammering G., De Ruysscher D.K., Lambin P. Combined use of hyperthermia and radiation therapy for treating locally advanced cervix carcinoma. Cochrane Database Syst. Rev. 2010;3:CD006377. doi: 10.1002/14651858.CD006377.pub3. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

