GABAergic Input Affects Intracellular Calcium Levels in Developing Granule Cells of Adult Rat Hippocampus
- PMID: 32138257
- PMCID: PMC7084234
- DOI: 10.3390/ijms21051715
GABAergic Input Affects Intracellular Calcium Levels in Developing Granule Cells of Adult Rat Hippocampus
Abstract
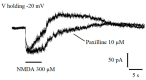
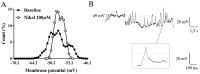
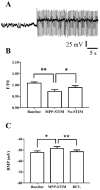
In the dentate gyrus (DG) of the mammalian hippocampus, granule neurons are generated from neural stem cells (NSCs) throughout the life span and are integrated into the hippocampal network. Adult DG neurogenesis is regulated by multiple intrinsic and extrinsic factors that control NSC proliferation, maintenance, and differentiation into mature neurons. γ-Aminobutyric acid (GABA), released by local interneurons, regulates the development of neurons born in adulthood by activating extrasynaptic and synaptic GABAA receptors. In the present work, patch-clamp and calcium imaging techniques were used to record very immature granule cells of adult rat dentate gyrus for investigating the actual role of GABAA receptor activation in intracellular calcium level regulation at an early stage of maturation. Our findings highlight a novel molecular and electrophysiological mechanism, involving calcium-activated potassium channels (BK) and T-type voltage-dependent calcium channels, through which GABA fine-tunes intracellular calcium homeostasis in rat adult-born granule neurons early during their maturation. This mechanism might be instrumental in promoting newborn cell survival.
Keywords: GABA input; T-type voltage-dependent calcium channels; adult rat; calcium-activated potassium channels; hippocampus; immature neurons; membrane potential oscillations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Ambrogini P., Lattanzi D., Ciuffoli S., Agostini D., Bertini L., Stocchi V., Santi S., Cuppini R. Morpho-functional characterization of neuronal cells at different stages of maturation in granule cell layer of adult rat dentate gyrus. Brain Res. 2004;1017:21–31. doi: 10.1016/j.brainres.2004.05.039. - DOI - PubMed
-
- Bao H., Asrican B., Li W., Gu B., Wen Z., Lim S.A., Haniff I., Ramakrishnan C., Deisseroth K., Philpot B., et al. Long-Range GABAergic Inputs Regulate Neural Stem Cell Quiescence and Control Adult Hippocampal Neurogenesis. Cell Stem Cell. 2017;21:604–617. doi: 10.1016/j.stem.2017.10.003. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

