Homocysteine Induces Inflammation in Retina and Brain
- PMID: 32138265
- PMCID: PMC7175372
- DOI: 10.3390/biom10030393
Homocysteine Induces Inflammation in Retina and Brain
Abstract
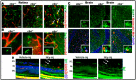
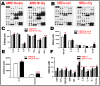
Homocysteine (Hcy) is an amino acid that requires vitamins B12 and folic acid for its metabolism. Vitamins B12 and folic acid deficiencies lead to hyperhomocysteinemia (HHcy, elevated Hcy), which is linked to the development of diabetic retinopathy (DR), age-related macular degeneration (AMD), and Alzheimer's disease (AD). The goal of the current study was to explore inflammation as an underlying mechanism of HHcy-induced pathology in age related diseases such as AMD, DR, and AD. Mice with HHcy due to a lack of the enzyme cystathionine-β-synthase (CBS) and wild-type mice were evaluated for microglia activation and inflammatory markers using immuno-fluorescence (IF). Tissue lysates isolated from the brain hippocampal area from mice with HHcy were evaluated for inflammatory cytokines using the multiplex assay. Human retinal endothelial cells, retinal pigment epithelial cells, and monocyte cell lines treated with/without Hcy were evaluated for inflammatory cytokines and NFκB activation using the multiplex assay, western blot analysis, and IF. HHcy induced inflammatory responses in mouse brain, retina, cultured retinal, and microglial cells. NFκB was activated and cytokine array analysis showed marked increase in pro-inflammatory cytokines and downregulation of anti-inflammatory cytokines. Therefore, elimination of excess Hcy or reduction of inflammation is a promising intervention for mitigating damage associated with HHcy in aging diseases such as DR, AMD, and AD.
Keywords: Alzheimer’s disease; age-related macular degeneration; diabetic retinopathy; homocysteine; inflammation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

