MicroRNA-24-3p Targets Notch and Other Vascular Morphogens to Regulate Post-ischemic Microvascular Responses in Limb Muscles
- PMID: 32138369
- PMCID: PMC7084374
- DOI: 10.3390/ijms21051733
MicroRNA-24-3p Targets Notch and Other Vascular Morphogens to Regulate Post-ischemic Microvascular Responses in Limb Muscles
Abstract
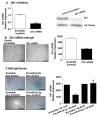
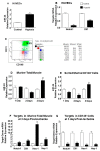
MicroRNAs (miRs) regulate complex processes, including angiogenesis, by targeting multiple mRNAs. miR-24-3p-3p directly represses eNOS, GATA2, and PAK4 in endothelial cells (ECs), thus inhibiting angiogenesis during development and in the infarcted heart. miR-24-3p is widely expressed in cardiovascular cells, suggesting that it could additionally regulate angiogenesis by acting on vascular mural cells. Here, we have investigated: 1) new miR-24-3p targets; 2) the expression and the function of miR-24-3p in human vascular ECs; 3) the impact of miR-24-3p inhibition in the angiogenesis reparative response to limb ischemia in mice. Using bioinformatics target prediction platforms and 3'-UTR luciferase assays, we newly identified Notch1 and its Delta-like ligand 1 (Dll1) to be directly targeted by miR-24-3p. miR-24-3p was expressed in human ECs and pericytes cultured under normal conditions. Exposure to hypoxia increased miR-24-3p in ECs but not in pericytes. Transfection with a miR-24-3p precursor (pre-miR-24-3p) increased miR-24-3p expression in ECs, reducing the cell survival, proliferation, and angiogenic capacity. Opposite effects were caused by miR-24-3p inhibition. The anti-angiogenic action of miR-24-3p overexpression could be prevented by simultaneous adenovirus (Ad)-mediated delivery of constitutively active Notch intracellular domain (NICD) into cultured ECs. We next demonstrated that reduced Notch signalling contributes to the anti-angiogenic effect of miR-24-3p in vitro. In a mouse unilateral limb ischemia model, local miR-24-3p inhibition (by adenovirus-mediated miR-24-3p decoy delivery) restored endothelial Notch signalling and increased capillary density. However, the new vessels appeared disorganised and twisted, worsening post-ischemic blood perfusion recovery. To better understand the underpinning mechanisms, we widened the search for miR-24-3p target genes, identifying several contributors to vascular morphogenesis, such as several members of the Wingless (Wnt) signalling pathway, β-catenin signalling components, and VE-cadherin, which synergise to regulate angiogenesis, pericytes recruitment to neoformed capillaries, maturation, and stabilization of newly formed vessels. Among those, we next focussed on β-catenin to demonstrate that miR-24-3p inhibition reduces β-catenin expression in hypoxic ECs, which is accompanied by reduced adhesion of pericytes to ECs. In summary, miR-24-3p differentially targets several angiogenesis modulators and contributes to autonomous and non-autonomous EC crosstalk. In ischemic limbs, miR-24-3p inhibition increases the production of dysfunctional microvessels, impairing perfusion. Caution should be observed in therapeutic targeting of miR-24-3p.
Keywords: Notch; angiogenesis; endothelial cells; limb ischemia; miR-24-3p; β-catenin.
Conflict of interest statement
The authors have no conflicting financial interest.
Figures







Similar articles
-
miR-342-5p Is a Notch Downstream Molecule and Regulates Multiple Angiogenic Pathways Including Notch, Vascular Endothelial Growth Factor and Transforming Growth Factor β Signaling.J Am Heart Assoc. 2016 Feb 8;5(2):e003042. doi: 10.1161/JAHA.115.003042. J Am Heart Assoc. 2016. PMID: 26857067 Free PMC article.
-
Therapeutic Angiogenesis by Ultrasound-Mediated MicroRNA-126-3p Delivery.Arterioscler Thromb Vasc Biol. 2015 Nov;35(11):2401-11. doi: 10.1161/ATVBAHA.115.306506. Epub 2015 Sep 17. Arterioscler Thromb Vasc Biol. 2015. PMID: 26381870
-
Bach1 Represses Wnt/β-Catenin Signaling and Angiogenesis.Circ Res. 2015 Jul 31;117(4):364-375. doi: 10.1161/CIRCRESAHA.115.306829. Epub 2015 Jun 29. Circ Res. 2015. PMID: 26123998 Free PMC article.
-
Notch signalling in ischaemia-induced angiogenesis.Biochem Soc Trans. 2009 Dec;37(Pt 6):1221-7. doi: 10.1042/BST0371221. Biochem Soc Trans. 2009. PMID: 19909251 Free PMC article. Review.
-
MicroRNAs in vascular tissue engineering and post-ischemic neovascularization.Adv Drug Deliv Rev. 2015 Jul 1;88:78-91. doi: 10.1016/j.addr.2015.05.003. Epub 2015 May 14. Adv Drug Deliv Rev. 2015. PMID: 25980937 Free PMC article. Review.
Cited by
-
The possible role of SRMS in colorectal cancer by bioinformatics analysis.World J Surg Oncol. 2021 Nov 16;19(1):326. doi: 10.1186/s12957-021-02431-y. World J Surg Oncol. 2021. PMID: 34781983 Free PMC article.
-
Converging Effects of Three Different Endocrine Disrupters on Sox and Pou Gene Expression in Developing Rat Hippocampus: Possible Role of microRNA in Sex Differences.Front Genet. 2021 Nov 11;12:718796. doi: 10.3389/fgene.2021.718796. eCollection 2021. Front Genet. 2021. PMID: 34858468 Free PMC article.
-
Adding a "Notch" to Cardiovascular Disease Therapeutics: A MicroRNA-Based Approach.Front Cell Dev Biol. 2021 Aug 30;9:695114. doi: 10.3389/fcell.2021.695114. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34527667 Free PMC article. Review.
-
A nested case-control study of circular ribonucleic acid expression profiles in the peripheral blood of pregnant women with pre-eclampsia.Pak J Med Sci. 2024 Dec;40(11):2658-2664. doi: 10.12669/pjms.40.11.10317. Pak J Med Sci. 2024. PMID: 39634903 Free PMC article.
-
The Notch signaling-regulated angiogenesis in rheumatoid arthritis: pathogenic mechanisms and therapeutic potentials.Front Immunol. 2023 Oct 26;14:1272133. doi: 10.3389/fimmu.2023.1272133. eCollection 2023. Front Immunol. 2023. PMID: 38022508 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

