Transient Receptor Potential Canonical (TRPC) Channels as Modulators of Migration and Invasion
- PMID: 32138386
- PMCID: PMC7084769
- DOI: 10.3390/ijms21051739
Transient Receptor Potential Canonical (TRPC) Channels as Modulators of Migration and Invasion
Abstract
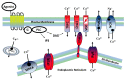
Calcium (Ca2+) is perhaps the most versatile signaling molecule in cells. Ca2+ regulates a large number of key events in cells, ranging from gene transcription, motility, and contraction, to energy production and channel gating. To accomplish all these different functions, a multitude of channels, pumps, and transporters are necessary. A group of channels participating in these processes is the transient receptor potential (TRP) family of cation channels. These channels are divided into 29 subfamilies, and are differentially expressed in man, rodents, worms, and flies. One of these subfamilies is the transient receptor potential canonical (TRPC) family of channels. This ion channel family comprises of seven isoforms, labeled TRPC1-7. In man, six functional forms are expressed (TRPC1, TRPC3-7), whereas TRPC2 is a pseudogene; thus, not functionally expressed. In this review, we will describe the importance of the TRPC channels and their interacting molecular partners in the etiology of cancer, particularly in regard to regulating migration and invasion.
Keywords: TRPC; angiogenesis; calcium; cancer; invasion; ion channels; migration; thyroid.
Conflict of interest statement
The Authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

