Viral Vectors, Animal Models, and Cellular Targets for Gene Therapy of Cystic Fibrosis Lung Disease
- PMID: 32138545
- PMCID: PMC7232698
- DOI: 10.1089/hum.2020.013
Viral Vectors, Animal Models, and Cellular Targets for Gene Therapy of Cystic Fibrosis Lung Disease
Abstract
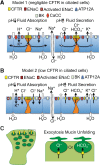
After more than two decades since clinical trials tested the first use of recombinant adeno-associated virus (rAAV) to treat cystic fibrosis (CF) lung disease, gene therapy for this disorder has undergone a tremendous resurgence. Fueling this enthusiasm has been an enhanced understanding of rAAV transduction biology and cellular processes that limit transduction of airway epithelia, the development of new rAAV serotypes and other vector systems with high-level tropism for airway epithelial cells, an improved understanding of CF lung pathogenesis and the cellular targets for gene therapy, and the development of new animal models that reproduce the human CF disease phenotype. These advances have created a preclinical path for both assessing the efficacy of gene therapies in the CF lung and interrogating the target cell types in the lung required for complementation of the CF disease state. Lessons learned from early gene therapy attempts with rAAV in the CF lung have guided thinking for the testing of next-generation vector systems. Although unknown questions still remain regarding the cellular targets in the lung that are required or sufficient to complement CF lung disease, the field is now well positioned to tackle these challenges. This review will highlight the role that next-generation CF animal models are playing in the preclinical development of gene therapies for CF lung disease and the knowledge gaps in disease pathophysiology that these models are attempting to fill.
Keywords: animal models; cellular targets; cystic fibrosis; gene therapy; pathophysiology; viral vectors.
Conflict of interest statement
Z.Y. and J.F.E. receive income for consulting with Spirovant Science. J.F.E. has sponsored research with Vertex Pharmaceuticals and Spirovant Science.
Figures

References
-
- Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 1989;245:1066–1073 - PubMed
-
- Kunzelmann K, Schreiber R, Hadorn HB. Bicarbonate in cystic fibrosis. J Cyst Fibros 2017;16:653–662 - PubMed
-
- Wine JJ, Hansson GC, Konig P, et al. Progress in understanding mucus abnormalities in cystic fibrosis airways. J Cyst Fibros 2018;17:S35–S39 - PubMed
-
- Zhou-Suckow Z, Duerr J, Hagner M, et al. Airway mucus, inflammation and remodeling: emerging links in the pathogenesis of chronic lung diseases. Cell Tissue Res 2017;367:537–550 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

