Severity of coeliac disease and clinical management study when using a CYP3A4 metabolised medication: a phase I pharmacokinetic study
- PMID: 32139488
- PMCID: PMC7059485
- DOI: 10.1136/bmjopen-2019-034086
Severity of coeliac disease and clinical management study when using a CYP3A4 metabolised medication: a phase I pharmacokinetic study
Abstract
Objective: Severity of coeliac disease depends in part on the extent of small intestinal mucosa injury. Patients with the most abnormal pathology have loss of duodenal villi CYP3A4, a drug-metabolising enzyme that inactivates many drugs. These patients are hypothesised to have greater systemic concentrations of felodipine, a drug which normally has low oral bioavailability secondary to intestinal CYP3A4-mediated metabolism. It serves as a representative for a class containing many medications.
Design: A phase I, open-label, single-dose, pharmacokinetic study.
Setting: London, Ontario, Canada.
Participants: Patients with coeliac disease (n=47) with positive serology and healthy individuals (n=68).
Main outcome measures: Patients with coeliac disease-upper gastrointestinal endoscopy and oral felodipine pharmacokinetics study within a 3-week period. Healthy individuals-oral felodipine pharmacokinetics study with water and grapefruit juice.
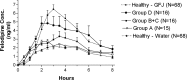
Results: Coeliac stratification categories: Group A (n=15, normal), B+C (n=16, intraepithelial lymphocytosis with/without mild villous blunting) and D (n=16, moderate/severe villous blunting). Groups A, B+C and D had linear trends of increasing felodipine AUC0-8; mean±SEM, 14.4±2.1, 17.6±2.8, 25.7±5.0; p<0.05) and Cmax (3.5±0.5, 4.0±0.6, 6.4±1.1; p<0.02), respectively. Healthy subjects receiving water had lower felodipine AUC0-8 (11.9±0.9 vs 26.9±0.9, p=0.0001) and Cmax (2.9±0.2 vs 7.7±0.2, p=0.0001) relative to those receiving grapefruit juice.
Conclusions: Increased felodipine concentrations in patients with coeliac disease were most probably secondary to decreased small intestinal CYP3A4 expression. Patients with severe coeliac disease and healthy individuals with grapefruit juice had equivalently enhanced effect. Thus, patients with severe coeliac disease would probably experience similarly altered drug response, including overdose toxicity, from many important medications known to be metabolised by CYP3A4. Patients with coeliac disease with severe disease should be considered for other clinical drug management, particularly when there is the potential for serious drug toxicity.
Keywords: adverse events; clinical pharmacology; coeliac disease; gastroenterology.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Severity of coeliac disease and clinical management study when using a non-metabolised medication: a phase I pharmacokinetic study.BMJ Open. 2023 Feb 24;13(2):e057151. doi: 10.1136/bmjopen-2021-057151. BMJ Open. 2023. PMID: 36828648 Free PMC article. Clinical Trial.
-
Coffee inhibition of CYP3A4 in vitro was not translated to a grapefruit-like pharmacokinetic interaction clinically.Pharmacol Res Perspect. 2017 Oct;5(5):e00346. doi: 10.1002/prp2.346. Pharmacol Res Perspect. 2017. PMID: 28971609 Free PMC article. Clinical Trial.
-
Grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP3A protein expression.J Clin Invest. 1997 May 15;99(10):2545-53. doi: 10.1172/JCI119439. J Clin Invest. 1997. PMID: 9153299 Free PMC article.
-
Interactions between grapefruit juice and cardiovascular drugs.Am J Cardiovasc Drugs. 2004;4(5):281-97. doi: 10.2165/00129784-200404050-00002. Am J Cardiovasc Drugs. 2004. PMID: 15449971 Review.
-
Grapefruit juice and drug interactions. Exploring mechanisms of this interaction and potential toxicity for certain drugs.Geriatrics. 2006 Nov;61(11):12-8. Geriatrics. 2006. PMID: 17112309 Review.
Cited by
-
Guidelines for best practices in monitoring established coeliac disease in adult patients.Nat Rev Gastroenterol Hepatol. 2024 Mar;21(3):198-215. doi: 10.1038/s41575-023-00872-2. Epub 2023 Dec 18. Nat Rev Gastroenterol Hepatol. 2024. PMID: 38110546 Review.
-
Physiologically based pharmacokinetic modeling for development and applications of a virtual celiac disease population using felodipine as a model drug.CPT Pharmacometrics Syst Pharmacol. 2023 Jun;12(6):808-820. doi: 10.1002/psp4.12954. Epub 2023 Apr 2. CPT Pharmacometrics Syst Pharmacol. 2023. PMID: 36855819 Free PMC article.
-
Severity of coeliac disease and clinical management study when using a non-metabolised medication: a phase I pharmacokinetic study.BMJ Open. 2023 Feb 24;13(2):e057151. doi: 10.1136/bmjopen-2021-057151. BMJ Open. 2023. PMID: 36828648 Free PMC article. Clinical Trial.
-
Molecular Biomarkers for Celiac Disease: Past, Present and Future.Int J Mol Sci. 2020 Nov 12;21(22):8528. doi: 10.3390/ijms21228528. Int J Mol Sci. 2020. PMID: 33198309 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical