A prolonged multispecies outbreak of IMP-6 carbapenemase-producing Enterobacterales due to horizontal transmission of the IncN plasmid
- PMID: 32139745
- PMCID: PMC7057946
- DOI: 10.1038/s41598-020-60659-2
A prolonged multispecies outbreak of IMP-6 carbapenemase-producing Enterobacterales due to horizontal transmission of the IncN plasmid
Abstract
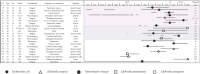
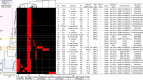
A multispecies outbreak of IMP-6 carbapenemase-producing Enterobacterales (IMP-6-CPE) occurred at an acute care hospital in Japan. This study was conducted to understand the mechanisms of IMP-6-CPE transmission by pulsed-field gel electrophoresis (PFGE), multilocus sequence typing and whole-genome sequencing (WGS), and identify risk factors for IMP-6-CPE acquisition in patients who underwent abdominal surgery. Between July 2013 and March 2014, 22 hospitalized patients infected or colonized with IMP-6-CPE (Escherichia coli [n = 8], Klebsiella oxytoca [n = 5], Enterobacter cloacae [n = 5], Klebsiella pneumoniae [n = 3] and Klebsiella aerogenes [n = 1]) were identified. There were diverse PFGE profiles and sequence types (STs) in most of the species except for K. oxytoca. All isolates of K. oxytoca belonged to ST29 with similar PFGE profiles, suggesting their clonal transmission. Plasmid analysis by WGS revealed that all 22 isolates but one shared a ca. 50-kb IncN plasmid backbone with blaIMP-6 suggesting interspecies gene transmission, and typing of plasmids explained epidemiological links among cases. A case-control study showed pancreatoduodenectomy, changing drains in fluoroscopy room, continuous peritoneal lavage and enteric fistula were associated with IMP-6-CPE acquisition among the patients. Plasmid analysis of isolates in an outbreak of IMP-6-CPE suggested interspecies gene transmission and helped to clarify hidden epidemiological links between cases.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- WHO|Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. WHO. Available at, https://www.who.int/medicines/publications/global-priority-list-antibiot... (2017)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

