Lipogenic signalling modulates prostate cancer cell adhesion and migration via modification of Rho GTPases
- PMID: 32139877
- PMCID: PMC7190568
- DOI: 10.1038/s41388-020-1243-2
Lipogenic signalling modulates prostate cancer cell adhesion and migration via modification of Rho GTPases
Abstract
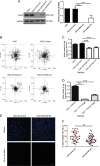
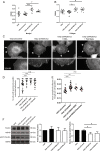
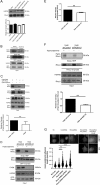
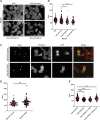
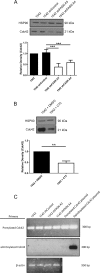
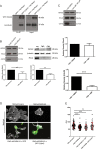
Fatty acid synthase (FASN) is commonly overexpressed in prostate cancer and associated with tumour progression. FASN is responsible for de novo synthesis of the fatty acid palmitate; the building block for protein palmitoylation. Recent work has suggested that alongside its established role in promoting cell proliferation FASN may also promote invasion. We now find depletion of FASN expression increases prostate cancer cell adhesiveness, impairs HGF-mediated cell migration and reduces 3D invasion. These changes in motility suggest that FASN can mediate actin cytoskeletal remodelling; a process known to be downstream of Rho family GTPases. Here, we demonstrate that modulation of FASN expression specifically impacts on the palmitoylation of the atypical GTPase RhoU. Impaired RhoU activity in FASN depleted cells leads to reduced adhesion turnover downstream of paxillin serine phosphorylation, which is rescued by addition of exogenous palmitate. Moreover, canonical Cdc42 expression is dependent on the palmitoylation status of RhoU. Thus we uncover a novel relationship between FASN, RhoU and Cdc42 that directly influences cell migration potential. These results provide compelling evidence that FASN activity directly promotes cell migration and supports FASN as a potential therapeutic target in metastatic prostate cancer.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- American Cancer Society. Survival rates for prostate cancer. American Cancer Society; 2016. http://www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

