Nanoengineered Advanced Materials for Enabling Hydrogen Economy: Functionalized Graphene-Incorporated Cupric Oxide Catalyst for Efficient Solar Hydrogen Production
- PMID: 32140256
- PMCID: PMC7050082
- DOI: 10.1002/gch2.201900087
Nanoengineered Advanced Materials for Enabling Hydrogen Economy: Functionalized Graphene-Incorporated Cupric Oxide Catalyst for Efficient Solar Hydrogen Production
Abstract
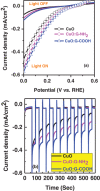
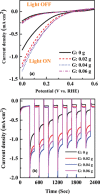
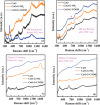
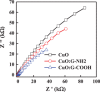
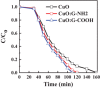
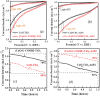
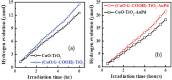
Cupric oxide (CuO) is a promising candidate as a photocathode for visible-light-driven photo-electrochemical (PEC) water splitting. However, the stability of the CuO photocathode against photo-corrosion is crucial for developing CuO-based PEC cells. This study demonstrates a stable and efficient photocathode through the introduction of graphene into CuO film (CuO:G). The CuO:G composite electrodes are prepared using graphene-incorporated CuO sol-gel solution via spin-coating techniques. The graphene is modified with two different types of functional groups, such as amine (-NH2) and carboxylic acid (-COOH). The -COOH-functionalized graphene incorporation into CuO photocathode exhibits better stability and also improves the photocurrent generation compare to control CuO electrode. In addition, -COOH-functionalized graphene reduces the conversion of CuO phase into cuprous oxide (Cu2O) during photo-electrochemical reaction due to effective charge transfer and leads to a more stable photocathode. The reduction of CuO to Cu2O phase is significantly lesser in CuO:G-COOH as compared to CuO and CuO:G-NH2 photocathodes. The photocatalytic degradation of methylene blue (MB) by CuO, CuO:G-NH2 and CuO:G-COOH is also investigated. By integrating CuO:G-COOH photocathode with a sol-gel-deposited TiO2 protecting layer and Au-Pd nanostructure, stable and efficient photocathode are developed for solar hydrogen generation.
Keywords: Raman spectroscopy; photocatalytic degradation; photocorrosion stability; solar hydrogen.
© 2020 The Authors. Published by WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Maeda K., Domen K., J. Phys. Chem. Lett. 2010, 1, 2655.
-
- Dubale A. A., Tamirat A. G., Chen H.‐M., Berhe T. A., Pan C.‐J., Su W.‐N., Hwang B.‐J., J. Mater. Chem. A 2016, 4, 2205.
-
- Wang C., Ma B., Xu S., Li D., He S., Zhao Y., Han J., Wei M., Evans D. G., Duan X., Nano Energy 2017, 32, 463.
-
- Fàbrega C., Murcia‐López S., Monllor‐Satoca D., Prades J. D., Hernández‐Alonso M. D., Penelas G., Morante J. R., Andreua T., Appl. Catal., B 2016, 189, 133. - PubMed
-
- Djurišić A. B., Leung Y. H., Ng A. M. C., Mater. Horiz. 2014, 1, 400.
LinkOut - more resources
Full Text Sources
Miscellaneous

