The special considerations of gene therapy for mitochondrial diseases
- PMID: 32140258
- PMCID: PMC7051955
- DOI: 10.1038/s41525-020-0116-5
The special considerations of gene therapy for mitochondrial diseases
Abstract
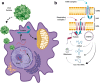
The recent success of gene therapy across multiple clinical trials has inspired a great deal of hope regarding the treatment of previously intractable genetic diseases. This optimism has been extended to the prospect of gene therapy for mitochondrial disorders, which are not only particularly severe but also difficult to treat. However, this hope must be tempered by the reality of the mitochondrial organelle, which possesses specific biological properties that complicate genetic manipulation. In this perspective, we will discuss some of these complicating factors, including the unique pathways used to express and import mitochondrial proteins. We will also present some ways in which these challenges can be overcome by genetic manipulation strategies tailored specifically for mitochondrial diseases.
Keywords: Diseases; Genetics research; Medical genetics.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

