Cold Formalin Fixation Guarantees DNA Integrity in Formalin Fixed Paraffin Embedded Tissues: Premises for a Better Quality of Diagnostic and Experimental Pathology With a Specific Impact on Breast Cancer
- PMID: 32140450
- PMCID: PMC7042205
- DOI: 10.3389/fonc.2020.00173
Cold Formalin Fixation Guarantees DNA Integrity in Formalin Fixed Paraffin Embedded Tissues: Premises for a Better Quality of Diagnostic and Experimental Pathology With a Specific Impact on Breast Cancer
Abstract
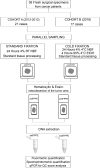
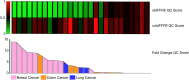
Formalin fixation and paraffin embedding (FFPE) represent the standard method to preserve tissue specimens for diagnostic pathology, however formalin fixation induces severe fragmentation of nucleic acids. We investigated whether formalin fixation at 4°C could preserve DNA integrity in FFPE specimens. Paired samples from 38 specimens were formalin fixed at room temperature (stdFFPE) and at 4°C (coldFFPE), respectively. Two independent cohorts were prospectively collected, cohort A (collected 6 years prior to the study, n = 21), cohort B (collected at time of the study, n = 17). DNA was extracted and its integrity evaluated with a qPCR-based assay that produces a normalized integrity index, the QC score (ratio between the quantity of a long and a short amplicon of the same gene). We observed higher QC scores in coldFFPE compared to stdFFPE samples (mean values: 0.69 vs. 0.36, p < 0.0001) and stdFFPE breast cancer specimens showed the most detrimental effect overall. Comparable QC scores were obtained between coldFFPE tissues of both cohorts; conversely, DNA integrity of stdFFPE was significantly lower in cohort A compared to cohort B (p < 0.0001). Of note, QC scores of stdFFPE (but not of coldFFPE) samples were significantly reduced following 6 months of storage (p = 0.0001). Monitored formalin fixation at 4°C outperforms standard fixation in ensuring high-quality DNA, which is key to feasibility of downstream high-throughput molecular analyses. An important effect was observed over storage time, thus suggesting a likely better preservation of archival samples when this cold fixation protocol is used.
Keywords: DNA fragmentation; biomarkers; breast cancer; cold formalin; diagnostic accuracy; fixation; molecular diagnostics; oncology.
Copyright © 2020 Berrino, Annaratone, Miglio, Maldi, Piccinelli, Peano, Balmativola, Cassoni, Pisacane, Sarotto, Venesio, Sapino and Marchiò.
Figures


References
-
- Hedegaard J, Thorsen K, Lund MK, Hein AM, Hamilton-Dutoit SJ, Vang S, et al. . Next-generation sequencing of RNA and DNA isolated from paired fresh-frozen and formalin-fixed paraffin-embedded samples of human cancer and normal tissue. PLoS ONE. (2014) 9:e98187. 10.1371/journal.pone.0098187 - DOI - PMC - PubMed

