Neuron-specific knockdown of solute carrier protein SLC25A46a induces locomotive defects, an abnormal neuron terminal morphology, learning disability, and shortened lifespan
- PMID: 32140609
- PMCID: PMC7047145
- DOI: 10.1016/j.ibror.2020.02.001
Neuron-specific knockdown of solute carrier protein SLC25A46a induces locomotive defects, an abnormal neuron terminal morphology, learning disability, and shortened lifespan
Abstract
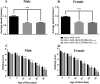
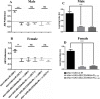
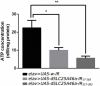
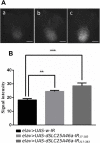
Various mutations in the SLC25A46 gene have been reported in mitochondrial diseases that are sometimes classified as type 2 Charcot-Marie-Tooth disease, optic atrophy, and Leigh syndrome. Although human SLC25A46 is a well-known transporter that acts through the mitochondrial outer membrane, the relationship between neurodegeneration in these diseases and the loss-of-function of SLC25A46 remains unclear. Two Drosophila genes, CG8931 (dSLC25A46a) and CG5755 (dSLC25A46b) have been identified as candidate homologs of human SLC25A46. We previously characterized the phenotypes of pan-neuron-specific dSLC25A46b knockdown flies. In the present study, we developed pan-neuron-specific dSLC25A46a knockdown flies and examined their phenotypes. Neuron-specific dSLC25A46a knockdown resulted in reduced mobility in larvae as well as adults. An aberrant morphology for neuromuscular junctions (NMJs), such as a reduced synaptic branch length and decreased number and size of boutons, was observed in dSLC25A46a knockdown flies. Learning ability was also reduced in the larvae of knockdown flies. In dSLC25A46a knockdown flies, mitochondrial hyperfusion was detected in NMJ synapses together with the accumulation of reactive oxygen species and reductions in ATP. These phenotypes were very similar to those of dSLC25A46b knockdown flies, suggesting that dSLC25A46a and dSLC25A46b do not have redundant roles in neurons. Collectively, these results show that the depletion of SLC25A46a leads to mitochondrial defects followed by an aberrant synaptic morphology, resulting in locomotive defects and learning disability. Thus, the dSLC25A46a knockdown fly summarizes most of the phenotypes in patients with mitochondrial diseases, offering a useful tool for studying these diseases.
Keywords: Drosophila; Learning abilities; Mitochondria; Neuromuscular junction; dSLC25A46.
© 2020 The Author(s).
Figures





References
-
- Abrams A.J., Hufnagel R.B., Rebelo A., Zanna C., Patel N., Gonzalez M.A., Campeanu I.J., Griffin L.B., Groenewald S., Strickland A.V., Tao F., Speziani F., Abreu L., Schüle R., Caporali L., La Morgia C., Maresca A., Liguori R., Lodi R., Ahmed Z.M., Sund K.L., Wang X., Krueger L.A., Peng Y., Prada C.E., Prows C.A., Schorry E.K., Antonellis A., Zimmerman H.H., Abdul-Rahman O.A., Yang Y., Downes S.M., Prince J., Fontanesi F., Barrientos A., Németh A.H., Carelli V., Huang T., Zuchner S., Dallman J.E. Mutations in SLC25A46, encoding a UGO1-like protein, cause an optic atrophy spectrum disorder. Nat. Genet. 2015;47:926–932. - PMC - PubMed
-
- Altanbyek V., Cha S.-J., Kang G.-U., Im D.S., Lee S., Kim H.-J., Kim K. Imbalance of mitochondrial dynamics in Drosophila models of amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 2016;481:259–264. - PubMed
-
- Azuma Y., Tokuda T., Shimamura M., Kyotani A., Sasayama H., Yoshida T., Mizuta I., Mizuno T., Nakagawa M., Fujikake N., Ueyama M., Nagai Y., Yamaguchi M. Identification of ter94, Drosophila VCP, as a strong modulator of motor neuron degeneration induced by knockdown of Caz, Drosophila FUS. Hum. Mol. Genet. 2014;23:3467–3480. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

