Pharmacological targeting of α3β4 nicotinic receptors improves peripheral insulin sensitivity in mice with diet-induced obesity
- PMID: 32140744
- PMCID: PMC7228898
- DOI: 10.1007/s00125-020-05117-4
Pharmacological targeting of α3β4 nicotinic receptors improves peripheral insulin sensitivity in mice with diet-induced obesity
Abstract
Aims/hypothesis: Treatment with the α3β4 nicotinic acetylcholine receptor (nAChR) agonist, 1,1-dimethyl-4-phenylpiperazinium iodide (DMPP), improves glucose tolerance in diet-induced obese (DIO) mice, but the physiological and molecular mechanisms are unknown.
Methods: DMPP (10 mg/kg body weight, s.c.) was administered either in a single injection (acute) or daily for up to 14 days (chronic) in DIO wild-type (WT) and Chrnb4 knockout (KO) mice and glucose tolerance, tissue-specific tracer-based glucose metabolism, and insulin signalling were assessed.
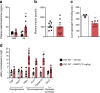
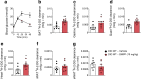
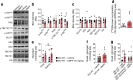
Results: In WT mice, but not in Chrnb4 KO mice, single acute treatment with DMPP induced transient hyperglycaemia, which was accompanied by high plasma adrenaline (epinephrine) levels, upregulated hepatic gluconeogenic genes, and decreased hepatic glycogen content. In contrast to these acute effects, chronic DMPP treatment in WT mice elicited improvements in glucose tolerance already evident after three consecutive days of DMPP treatment. After seven days of DMPP treatment, glucose tolerance was markedly improved, also in comparison with mice that were pair-fed to DMPP-treated mice. The glycaemic benefit of chronic DMPP was absent in Chrnb4 KO mice. Chronic DMPP increased insulin-stimulated glucose clearance into brown adipose tissue (+69%), heart (+93%), gastrocnemius muscle (+74%) and quadriceps muscle (+59%), with no effect in white adipose tissues. After chronic DMPP treatment, plasma adrenaline levels did not increase following an injection with DMPP. In glucose-stimulated skeletal muscle, we detected a decreased phosphorylation of the inhibitory Ser640 phosphorylation site on glycogen synthase and a congruent increase in glycogen accumulation following chronic DMPP treatment.
Conclusions/interpretation: Our data suggest that DMPP acutely induces adrenaline release and hepatic glycogenolysis, while chronic DMPP-mediated activation of β4-containing nAChRs improves peripheral insulin sensitivity independently of changes in body weight via mechanisms that could involve increased non-oxidative glucose disposal into skeletal muscle.
Keywords: Catecholamine; Glucose metabolism; Glucose tolerance; Hyperglycaemia; Insulin sensitivity; Nicotinic acetylcholine receptor; Pharmacology.
Figures


References
-
- Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. doi: 10.1016/s0140-6736(14)60460-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

