Submicrometer-Sized Roughness Suppresses Bacteria Adhesion
- PMID: 32142252
- PMCID: PMC7226781
- DOI: 10.1021/acsami.9b22621
Submicrometer-Sized Roughness Suppresses Bacteria Adhesion
Abstract
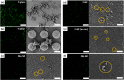
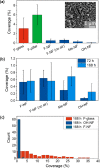
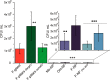
Biofilm formation is most commonly combatted with antibiotics or biocides. However, proven toxicity and increasing resistance of bacteria increase the need for alternative strategies to prevent adhesion of bacteria to surfaces. Chemical modification of the surfaces by tethering of functional polymer brushes or films provides a route toward antifouling coatings. Furthermore, nanorough or superhydrophobic surfaces can delay biofilm formation. Here we show that submicrometer-sized roughness can outweigh surface chemistry by testing the adhesion of E. coli to surfaces of different topography and wettability over long exposure times (>7 days). Gram-negative and positive bacterial strains are tested for comparison. We show that an irregular three-dimensional layer of silicone nanofilaments suppresses bacterial adhesion, both in the presence and absence of an air cushion. We hypothesize that a 3D topography can delay biofilm formation (i) if bacteria do not fit into the pores of the coating or (ii) if bending of the bacteria is required to adhere. Thus, such a 3D topography offers an underestimated possibility to design antibacterial surfaces that do not require biocides or antibiotics.
Keywords: antifouling; bacterial size; biofouling; roughness; silicone nanofilaments.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Enning D.; Venzlaff H.; Garrelfs J.; Dinh H. T.; Meyer V.; Mayrhofer K.; Hassel A. W.; Stratmann M.; Widdel F. Marine Sulfate-reducing Bacteria Cause Serious Corrosion of Iron under Electroconductive Biogenic Mineral Crust. Environ. Microbiol. 2012, 14, 1772–1787. 10.1111/j.1462-2920.2012.02778.x. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

